2976
Slice-Accelerated Inner-Volume Cervical Spinal Cord Diffusion MRI1Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
Cardiac-gated reduced-field of view (FOV) axial cervical spinal cord (C-spine) diffusion MRI (dMRI) has become a mainstay acquisition protocol to achieve tract-specific quantification of tissue microstructure in the C-spine. Building on our previous work in tract-specific C-spine dMRI, we summarize our experiences in combining slice-acceleration (i.e., multiband) with inner-volume-imaging (IVI) for quantitative C-spine dMRI at both 3T and 7T.
Introduction
Reduced FOV (rFOV) diffusion sequences1–7, using IVI and/or outer volume suppression techniques, produce high quality in vivo human C-spine dMRI images. However, lengthy acquisition time of cardiac-gated rFOV C-spine dMRI remains an impediment to clinical adoption where a large portion of the C-spine must be imaged. Here, we evaluate the combination of multiband (MB) slice-acceleration with IVI to achieve efficient coverage, while retaining high data quality for quantitative tract-specific C-spine dMRI.Methods
Subjects: Three healthy volunteers were recruited to participate in optimization and testing of imaging protocols. In addition, one subject was scanned three times at site 1 (Skyra) and two times at site 2 (Trio) as part of a reproducibility study 8,9.
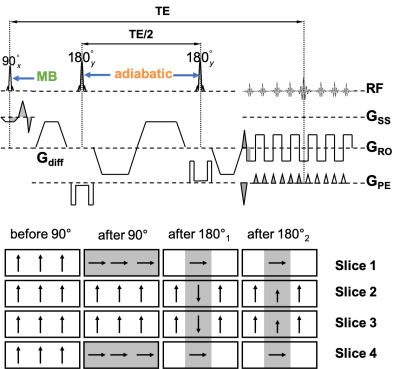
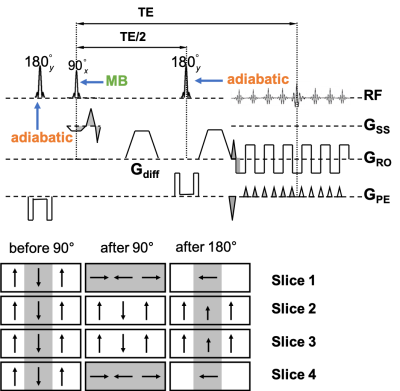
Sequence: Two schemes of the MB-IVI SE-EPI diffusion sequence were implemented. The first scheme (Fig. 1) uses twice-refocusing with bipolar diffusion encoding to minimize eddy currents, while the second scheme (Fig. 2) uses single-refoucusing with monopolar diffusion encoding to minimize echo time (TE). The double-inversion mechanism3 is retained in both schemes to preserve longitudinal magnetization and thereby maximize efficiency. In addition, hyperbolic secant (HSN) RF pulses were implemented for inversion and refocusing to optimize the slab profile in the phase-encoding (PE) direction across all excited slices in a single MB-pulse excitation. Since the refocusing pulses are applied in the PE direction, they need not be MB pulses.
Acquisitions: Axial MB-IVI dMRI protocols were implemented on four different Siemens platforms as part of ongoing studies: at 3 T (1) Skyra with HE4 + NC1/2 elements from a 20ch head/neck coil, (2) Trio with NC1/2 elements from a 4ch neck coil, (3) Prisma with HC7 + NC1/2 elements from a 64ch head/neck coil, and at 7T (4) Magnetom AS with a custom 22-channel brainstem/cervical spinal cord coil10. Imaging parameters: in-plane resolution 0.9mm×0.9mm for Skyra and Trio, 0.75mm×0.75mm for Prisma, and 0.60mm×0.60mm for Magnetom AS, 5mm slice thickness, 12 slices covering C2-C5, MB=2 with CAIPIRINHA shift=FOV/211, TE=65-75 ms depending on the scanner platform, anterior-posterior PE direction, monopolar diffusion encoding, multi-b values (linearly spaced, up to 1000s/mm2) and multi-b vectors12 (Fig. 2). In addition, during pilot acquisitions at 3T, residual aliasing, noise amplification, and image artifacts were compared between acquisitions with MB3 and MB2; while image distortion was evaluated between monopolar and bipolar schemes of the MB-IVI diffusion sequence using an MB2 protocol.
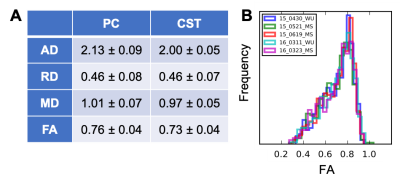
Analysis: For the reproducibility dataset, dMRI volumes were aligned using slice-wise x and y translations12. After excluding dMRI slice/volume containing image artifacts, DTI maps (including axial (AD), radial (RD), mean diffusivities (MD), and fractional anisotropy (FA)) were calculated in manually defined corticospinal tract (CST) and posterior column (PC) ROIs12.
Results and Discussion
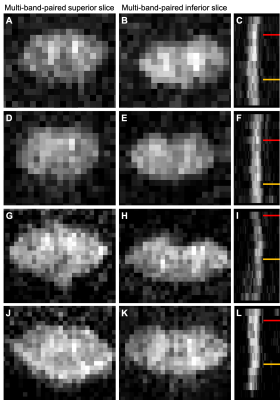
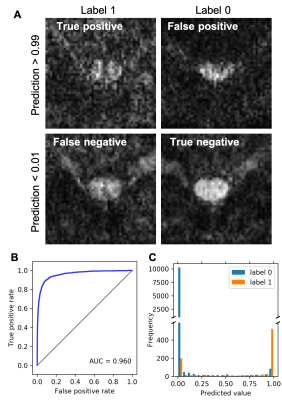
There was minimal difference in distortion between monopolar and bipolar schemes of the MB-IVI diffusion sequence (total acquired k-space lines = 36-48) at 3T, while image SNR was reduced for the bipolar diffusion encoding as expected (data not shown). Increased residual aliasing and g-factor noise amplification were observed in MB3 protocols, compared MB2 protocols at 3T (data not shown). Overall, MB2 protocols with CAIPIRINHA shift=FOV/2 yielded consistent high image quality across a variety of platforms (Fig. 3). Nevertheless, image artifacts (e.g., shading, signal dropout, pixelation, etc.) are more likely (~5%) to appear in diffusion-weighted images in the slice-accelerated than non-accelerated C-spine acquisition, probably because of the sensitivity of MB acquisition to high level of physiological noise at C-spine. To mitigate this issue, we have recently developed a machine-learning algorithm to facilitate efficient identification of these artifacts (Fig. 4)13. The reproducibility of the DTI measurements from a single subject is summarized in Fig. 5 as an example, while example C-spine DTI maps using MB-IVI acquisition will be presented in a separate ISMRM abstract9.Conclusion
For axial multi-slice C-spine dMRI covering a large portion of the C-spine, MB2 with CAIPIRINHA shift of FOV/2 allows for efficient cardiac-gated IVI acquisition across a variety of imaging platforms with minimal increases in g-factor noise or residual aliasing. Although there is an increased likelihood of image artifacts with MB acquisition, this minor detrimental effect, which can be identified and rejected from analysis using a machine-learning artifact detection algorithm, is outweighted by the 2-fold increase in acquisition efficiency.Acknowledgements
RSNA RSCH1328 (JX), International Progressive MS Alliance (IPMSA) infrastructure award PA0097, NINDS K01 NS105160 (ACS), NMSS FG-1606-24492 (J-WK).
References
1. Dowell, N. G., Jenkins, T. M., Ciccarelli, O., Miller, D. H. & Wheeler-Kingshott, C. A. M. Contiguous-slice zonally oblique multislice (CO-ZOOM) diffusion tensor imaging: Examples of in vivo spinal cord and optic nerve applications. J. Magn. Reson. Imaging 29, 454–460 (2009).
2. Finsterbusch, J. High‐resolution diffusion tensor imaging with inner field‐of‐view EPI. J. Magn. Reson. Imaging 29, 987–993 (2009).
3. Jeong, E. K., Kim, S. E., Guo, J. Y., Kholmovski, E. G. & Parker, D. L. High-resolution DTI with 2D interleaved multislice reduced FOV single-shot diffusion-weighted EPI (2D ss-rFOV-DWEPI). Magn. Reson. Med. 54, 1575–1579 (2005).
4. Kim, T. H. et al. Quantification of Diffusivities of the Human Cervical Spinal Cord Using a 2D Single-Shot Interleaved Multisection Inner Volume Diffusion-Weighted Echo-Planar Imaging Technique. Am. J. Neuroradiol. 31, 682–687 (2010).
5. Saritas, E. U., Cunningham, C. H., Lee, J. H., Han, E. T. & Nishimura, D. G. DWI of the spinal cord with reduced FOV single-shot EPI. Magn. Reson. Med. 60, 468–473 (2008).
6. Wheeler-Kingshott, C. A. M. et al. Investigating Cervical Spinal Cord Structure Using Axial Diffusion Tensor Imaging. NeuroImage 16, 93–102 (2002).
7. Wilm, B. J. et al. Reduced field-of-view MRI using outer volume suppression for spinal cord diffusion imaging. Magn. Reson. Med. 57, 625–630 (2007).
8. Kim, J.-W. et al. Mount Sinai - Washington University Quantitative Cervical Spinal Cord MRI Reproducibility Study Data. Proc. of the 27th Annual Meeting of ISMRM, Montreal, QC, Canada, (2019) submitted.
9. Kim, J.-W. et al. Reproducibility of Quantitative Cervical Spinal Cord MRI for Multi-center Clinical Trials in Multiple Sclerosis. Proc. of the 27th Annual Meeting of ISMRM, Montreal, QC, Canada, (2019) submitted.
10. Zhang, B., Seifert, A. C., Kim, J., Borrello, J. & Xu, J. 7 Tesla 22-channel wrap-around coil array for cervical spinal cord and brainstem imaging. Magn. Reson. Med. 78, 1623–1634 (2017).
11. Breuer, F. A. et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn. Reson. Med. 53, 684–691 (2005).
12. Xu, J. et al. Improved in vivo diffusion tensor imaging of human cervical spinal cord. NeuroImage 67, 64–76 (2013).
13. Kim, J.-W., Yang, C. & Xu, J. Automated spinal cord diffusion MRI quality assurance using deep neural network. ISMRM Workshop Mach. Learn. II (2019).
Figures