2771
Brain Microstructural Abnormalities in Patients with Idiopathic Rapid Eye Movement Sleep Behavior Disorder: A Voxel Based Morphometry and Diffusion Tensor Imaging Study1Department of Radiology, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China, 2Philips Healthcare, Guangzhou, China
Synopsis
Idiopathic rapid eye movement sleep behavior disorder (iRBD) has close relationship with neurodegenerative disorder. Many researches has validated that almost all of iRBD patients evolved into Parkinson’s disease, Lewy body dementia, or multiple system atrophy over time. However the pathogenesis of iRBD still remains unclear. In this research, VBM and voxel-based DTI analysis were combined to detect the microstructural abnormalities in the iRBD patients. A wide range of changes in brain structure in iRBD group was observed, which may reveal pathophysiologic mechanism on cognitive function disorder,which can be valuable for the early diagnosis and treatment of iRBD.
Purpose
Idiopathic rapid eye movement sleep behavior disorder (iRBD) is a parasomnia, which is characterized by dream-enacting behaviors and associated with loss of muscle atonia during REM sleep, but lack of any other neurological disease1. However, the specific pathogenesis of iRBD still remains unclear, which is considered as a risk factor for the development of neurodegenerative diseases, which is associated with alpha-synuclein deposition, such as Parkinson’s disease (PD), Lewy body dementia (LBD), and multiple system atrophy (MSA) 2. Few brain imaging studies have been performed to investigate the microstructural abnormalities involved in the same group of iRBD by using voxel based morphometry (VBM) and diffusion tensor imaging (DTI) together3-4. In this study, we explore the abnormal alterations in the brain of patients with iRBD by using VBM and DTI, trying to investigate the possible pathophysiological mechanisms of iRBD, as well as the relationship between iRBD and neurodegenerative diseases.Methods
The study protocol was approved by local research ethics committee. Written informed consent was obtained from each subject prior to participation this study. Fifteen patients with iRBD and twenty age- and gender-matched healthy controls were recruited in this study from January 2016 to July 2017. All the subjects were evaluated with the Mini-Mental State Exam (MMSE) on the same day with MRI examination5. To reduce the effects of pharmacological actions of nervous system associated drugs, all patients with iRBD were scanned in an OFF medication state, which was withdrawal from medication for more than 12 hours (The patients stopped the medication before 7:00 pm the day before the testing day to avoid disturbing for clinical therapy). The whole brain of all subjects was scanned using Philips 3.0 T MRI (Ingenia, Philips Healthcare, Best, The Netherlands). Raw data of 3D T1WI was preprocessed with SPM8, and DTI was subjected to a quality control (QC) using the DTIPrep tool (http://www.nitrc.org/plugins/mwiki/index.php/dtiprep:MainPage) to identify any artifacts on images, as well as to correct for motion and eddy current artifacts.The specific parameters include gray matter volume (GMV), white matter volume (WMV), fractional anisotropy (FA), mean diffusivity (MD) were calculated with software of SPM8(University College London, UK; http://www.fil.ion.ucl.ac.uk/spm) and PANDA(http://www.nitrc.org/projects/panda)6. A voxel-based comparison of FA and MD in whole brain was made between two groups. A threshold of P < 0.05 with AlphaSim correction and an extent voxel threshold of 85 were selected for determining the significant difference.Results
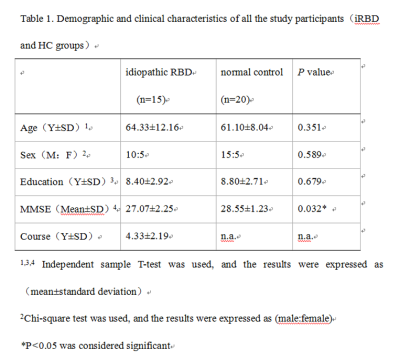
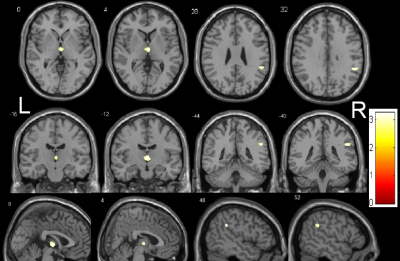
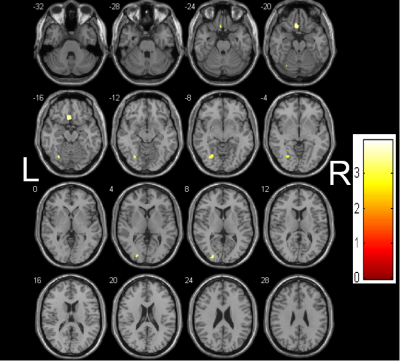
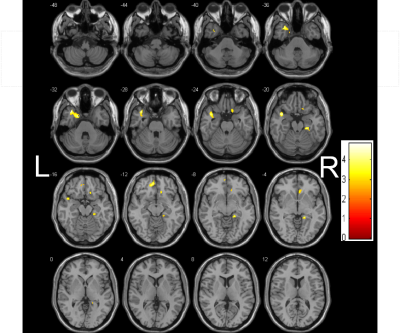
The demographic and clinical characteristics of the patients and controls are shown in Table 1. The MMSE score of iRBD group was lower than that of the healthy control group, and the difference was statistically significant. Decreased GMV in iRBD were detected in the regions of left thalamus, right supramarginal gyrus (Figure 1), and decreased WMV in the regions of left rectus gyrus, left fusiform gyrus, left middle occipital gyrus, left middle frontal gyrus compared with the healthy controls (Figure 2) (AlphaSim, p < 0.05, K ≥ 85 voxels). There were no regions with significantly increased GMV and WMV in iRBD. Increased MD in iRBD group versus controls was observed in the following regions: left superior temporal pole gyrus, left middle temporal pole gyrus, right parahippocampal gyrus, right rectus gyrus, left median frontal orbit gyrus(Figure 3). No regions with significantly decreased MD and abnormal FA of were noted in iRBD compared to controls.Conclusion:
Our results documented that patients with iRBD developed a wide range of changes in brain structure. The structural abnormalities of the brain in the frontal lobe, temporal lobe, occipital lobe, thalamus and parahippocampal are closely related with the occurrence of iRBD, possibly because of the damage in the regulatory loop of REM sleep, providing imaging evidence for the pathophysiological mechanism of iRBD. The abnormal alterations in the frontal lobe, parahippocampal are similar to the brain changes in parkinson's disease, multiple system atrophy and other nervous system degenerative diseases, suggesting that iRBD maybe correlated to nervous system degenerative diseases, as a possible precursor of neurodegenerative diseases.Acknowledgements
No Acknowledgement foundReferences
1. Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(2): 331-339.
2. Postuma RB, Gagnon JF, Montplaisir JY. REM sleep behavior disorder: from dreams to neurodegeneration. Neurobiology of Disease. 2012;46(3): 553-558.
3 Zhang T, Davatzikos C. Optimally-discriminative voxel-based analysis; proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, F, 2010 [C].
4 Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6): 805-821.
5. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3): 189-198.
6. Cui Z, Zhong S, Xu P, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Frontiers in Human Neuroscience. 2013, 7(42): 42.
Figures