2604
Elucidating the influence of healthy aging on white matter microstructure: A comparison of different diffusion MRI models1Department of Neurology, School of Medicine, Emory University, Atlanta, GA, United States, 2Department of Radiology and Imaging Sciences, School of Medicine, Emory University, Atlanta, GA, United States, 3Joint Department of BioMedical Engineering, Emory University and Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
To understand microstructural changes associated with healthy aging, multi-shell diffusion-weighted images were acquired in a group of 71 cognitively normal volunteers (31-young, 40-old). Signal representation and tissue specific models were used to assess relationship between age and WM microstructural changes. TBSS was performed for group-comparison. Results showed that FA and NODDI-based indices exhibited highest degree of sensitivity with overlap in much wider regions. The results also showed regional differences among FA and ODI. The influence of DKI was more regionalized and complemented by FA. The study demonstrated the sensitivity of higher-order models to the age-related changes in tissue microstructure.
Introduction
Quantifying the microstructure in adult human brain is necessary to improve our understanding on changes associated with normal aging and in disease states. With age, white matter (WM) integrity maybe compromised due to both myelin degeneration and axonal loss 1. Such changes in the tissue microstructural organization can be estimated using diffusion-MRI (dMRI) that probes water self-diffusion in tissue microenvironment 2. A number of studies have been done to study aging related changes using dMRI with signal representation schemes such as diffusion tensor imaging (DTI) and diffusion kurtosis imaging (DKI) 3,4. While sensitive to microstructural changes, these representations are not specific to the underlying biophysical processes. On the other hand, biophysical inspired models, such as neurite orientation dispersion and density imaging (NODDI) and its derivative, Bingham-NODDI have been proposed to be more specific to the underlying biophysical processes. However, to-date, only a few studies have explored the feasibility of tissue specific models in healthy aging studies 5, 6. The aim of the proposed study is to compare these dMRI methods in their assessment of age related changes in a group of cognitively normal subjects aged between 22 – 80 years.Material and Methods
Seventy one cognitively healthy subjects (31 young, ≤ 55 years, mean age 31.4 years and 40 old, > 55 years, mean age 63.7 years) were selected as a subset of the Emory Brain Imaging Project. One of the main criterion of the old subjects’ inclusion in the subset was their negative cerebrospinal fluid amyloid-β (Aβ -) status. The imaging was performed on a 3T MRI system (Siemens, Prisma) with a 32 channel head coil. The dMRI was acquired in the axial plane with a Multi-band EPI sequence (MB acceleration factor = 3). A multi-shell diffusion-weighting scheme was used with 3 b0, 10 b150, 10 b350, 64 b1000, 64 b2000, 64 b3000 and 104 b5000 diffusion weighting directions. The acquisition parameters were as follows: TR/TE = 2600/80 ms, 2 mm isotropic resolution, 69 slices. The raw diffusion-weighted images (DWI) were processed to reduce signal noise 7, 8, and effects from Gibbs ringing artefacts 9, subject motion 10, susceptibility induced artefacts 10 and B1 field inhomogeneity 11.
DTI based estimations were carried out by using DWI data with b=1000 s/mm2, whereas DKI was estimated using b=1000 and 2000 s/mm2. For NODDI and Bingham-NODDI, the entire range of b-values were used. For group-wises comparison, Tract based spatial statistics (TBSS) 12 was used. On the derived scalar skeletonized maps, permutation-based statistics were performed using 5000 permutation using the randomise tools in FSL with Threshold-Free Cluster Enhancement for multiple comparison correction. A corrected p-value of <0.05 was considered statistical significant.
Results and Discussion
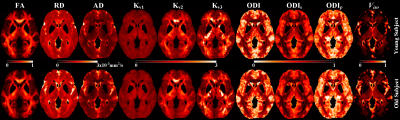
Figure 1 shows the representative maps of fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD), kurtosis along the principal eigenvector (Kv1), kurtosis along the secondary eigenvector (Kv2), kurtosis along the tertiary eigenvector (Kv3), orientation dispersion index (ODI) and volume fraction of isotropic water diffusion (Viso). Orientation dispersion along primary (ODIp) and secondary (ODIs) dispersion directions were estimated from Bingham-NODDI.
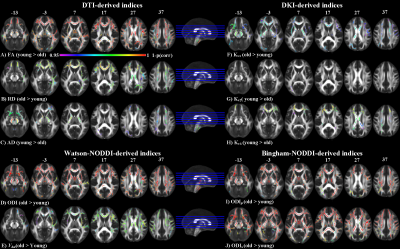
DTI based indices showed group-differences in major WM regions. FA expressed most widespread decrease in the elderly compared to the young groups with pronounced decrease in the genu of corpus callosum (CC). Increase in RD was also observed along these WM projections (Figure 2). DKI was less sensitive to age-related differences, with Kv1 showing decrease in kurtosis in the frontal WM and Kv3 showing increase in the same regions (Figure 2). ODI, Viso, ODIp and ODIs showed complementary differences (old > young) in most of the CC, internal capsule and corona radiata. ODIs also showed significant difference (old > young) in splenium and body of CC (Figure 2).
These results showed that ODI, Viso and FA displayed most widespread group differences, compared to the other derived indices. A high degree of overlap was also observed among these indices. Compared to FA, ODI also showed significant differences in the fornix and the internal capsule, whereas FA also showed difference in the splenium of CC.
Conclusion
The study showed the efficacy of dMRI to map the age-related differences in the microstructural environment of healthy adult WM. Compared to the signal representation based indices, which lack specificity, tissue specific model derived indices showed subtle differences in the microstructural composition. Thus, a combination of model free and tissue specific biophysical model can provide a better understanding of the underlying biophysiological processes and may be able to distinguish between mechanisms involved in healthy aging and disease.Acknowledgements
No acknowledgement found.References
1. Feldman, M.L. and Peters, A., 1998. Ballooning of myelin sheaths in normally aged macaques. Journal of neurocytology, 27(8), pp.605-614.
2. Callaghan, P.T. and Codd, S.L., 1998. Generalised calculation of NMR imaging edge effects arising from restricted diffusion in porous media. Magnetic resonance imaging, 16(5-6), pp.471-478.
3. Basser, P.J. and Pierpaoli, C., 2011. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance, 213(2), pp.560-570.
4. Jensen, J.H., Helpern, J.A., Ramani, A., Lu, H. and Kaczynski, K., 2005. Diffusional kurtosis imaging: the quantification of non‐gaussian water diffusion by means of magnetic resonance imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 53(6), pp.1432-1440.
5. Zhang, H., Schneider, T., Wheeler-Kingshott, C.A. and Alexander, D.C., 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 61(4), pp.1000-1016.
6. Tariq, M., Schneider, T., Alexander, D.C., Wheeler-Kingshott, C.A.G. and Zhang, H., 2016. Bingham–NODDI: mapping anisotropic orientation dispersion of neurites using diffusion MRI. NeuroImage, 133, pp.207-223.
7. Veraart, J., Novikov, D.S., Christiaens, D., Ades-Aron, B., Sijbers, J. and Fieremans, E., 2016. Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, pp.394-406.
8. Veraart, J., Fieremans, E. and Novikov, D.S., 2016. Diffusion MRI noise mapping using random matrix theory. Magnetic resonance in medicine, 76(5), pp.1582-1593.
9. Kellner, E., Dhital, B., Kiselev, V.G. and Reisert, M., 2016. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic resonance in medicine, 76(5), pp.1574-1581.
10. Andersson, J.L. and Sotiropoulos, S.N., 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, pp.1063-1078.
11. Zhang, Y., Brady, M. and Smith, S., 2001. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE transactions on medical imaging, 20(1), pp.45-57.
12. Smith, S.M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T.E., Mackay, C.E., Watkins, K.E., Ciccarelli, O., Cader, M.Z., Matthews, P.M. and Behrens, T.E., 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), pp.1487-1505.
Figures