2587
Free-Water Imaging Improves the Evaluation of White and Gray-Matter in Early Parkinson’s DiseaseChristina Andica1, Koji Kamagata1, Taku Hatano2, Yuki Takenaka1,3, Asami Saito1, Mana Kuramochi1,3, Wataru Uchida1,3, Akifumi Hagiwara1,4, Takashi Ogawa2, Haruka Takeshige-Amano2, Masaaki Hori1, Nobutaka Hattori2, and Shigeki Aoki1
1Department of Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Department of Neurology, Juntendo University School of Medicine, Tokyo, Japan, 3Department of Radiological Sciences, Tokyo Metropolitan University, Graduate School of Human Health Sciences, Tokyo, Japan, 4Department of Radiology, The University of Tokyo, Graduate School of Medicine, Tokyo, Japan
Synopsis
We applied bi-tensor diffusion model to evaluate the microstructural changes of white (WM) and gray matter (GM) in patients with early Parkinson’s disease (PDs). Our results demonstrated that the bi-tensor diffusion model could be used to disentangle neuroinflammation and axonal degeneration in early PDs with more precise estimations of localized microstructural changes compared to the single-tensor model. Our findings also suggest that microstructural changes in early PD may be preceded by neuroinflammation and followed by axonal degeneration, with WM changes preceding GM changes. Finally, the bi-tensor model also enabled to show possible compensatory mechanisms in PD occurring in the cerebellum.
Background and Purpose
Diffusion tensor imaging (DTI) has been widely used to investigate cerebral microstructural changes in Parkinson’s disease (PD), nonetheless with inconsistent results.1 One possible explanation is that diffusion MRI measurements are biased by partial volume effects from extracellular free-water (FW)2 and varying levels of FW in the tissue itself.3 The bi-tensor diffusion model was then introduced to quantify the contribution of FW and eliminates its bias on estimations of tissue microstructures, enabling the differentiation between alterations in the tissues themselves, as measured by the FW corrected fractional anisotropy (FAT), and extracellular FW changes, as measured by the fractional volume of FW.4 So far, FW imaging has been used to evaluate the substantia nigra of PD patients with promising results.5-9 This study aimed to comprehensively assess white matter (WM) and gray matter (GM) changes in early PD patients using single- and bi-tensor models of diffusion MRI.Materials and Methods
Twenty early PD patients (Hoehn & Yahr stage 1-2) and 20 healthy controls (HCs) (Table 1) were scanned on a 3T MR scanner and underwent 3D MPRAGE T1-WI and diffusion MRI consisting of b values of 0 and 1,000 s/mm2. Single-tensor FA and mean diffusivity (MD), and bi-tensor FAT, FW-corrected MD (MDT), and FW were computed. We used tract-based spatial statistics (TBSS) and gray matter-based spatial statistics (GBSS) to compare all indices in the WM and GM, respectively, between HCs and PDs, and to correlate the indices with disease duration and motor (Unified PD Rating Scale/UPDRS-III) and non-motor (UPDRS-I) severities in PD group. Further, we also performed voxel-based morphometry (VBM) for WM and GM volumes using the Statistical Parametric Mapping (SPM) 8 and cortical thickness analyses using the FreeSurfer 5.3.0.Results
- TBSS: significantly lower FA and higher MD were found in the broad area of WM in PDs compared to HCs. While significantly lower FAT and higher MDT were seen predominantly in the anterior and higher FW was seen in the posterior part of WM in PDs compared to HCs (Fig. 1).
- GBSS: significantly higher FW was found in a wide area of GM (Fig. 2) and higher FAT was found in the right cerebellum in PDs compared to HCs (Fig. 2). No significant difference was detected in FA and MD.
- Correlation tests of TBSS and GBSS: FW showed a positive correlation with UPDRS-I in the WM and GM (Fig. 3).
- VBM and cortical thickness: There were no significant differences in the WM and GM volumes and the cortical thickness between PD patients and HCs.
Discussion
In our study, changes in FAT and MDT in WM of early PD patients were shown in lesser extent areas compared with the single-tensor FA and MD. This is likely because single-tensor DTI metrics are not able to differentiate between WM degeneration and extracellular volume, while WM tracts that run in close adjacency to the ventricles are highly susceptible to CSF contamination.3 Lower FAT and higher MDT, which reflect axonal degeneration,3, 10 were observed predominantly in the anterior WM and higher FW, which reflects neuroinflammation,3, 10 was observed predominantly in the posterior WM in PDs compared to HCs. Considering that α-Synuclein, the major pathology of PD, has been shown to spread from the anterior to the posterior parts of WM,11 our findings suggest that axonal degeneration in PD might be preceded by neuroinflammation. These findings are supported by the fact that the chronic release of pro-inflammatory cytokines by activated astrocytes and microglia leads the exacerbation of dopaminergic neuron degeneration in the substantia nigra pars compacta.12 Further, higher FW was found in a wide area of GM in PDs compared to HCs. This might show that WM microstructural changes in early PD precede the changes of GM. Our findings are in agreement with previous studies, which showed widespread changes of WM microstructure in PD with limited GM alteration.13, 14 Finally, higher FAT in the cerebellum of PD patients compared to HCs might reflect the compensatory role of the cerebellum in PD.15 Previously, some studies demonstrated that the dysfunction of the striato-thalamo-cortical circuit is accompanied by the compensatory effect in the cerebello-thalamo-cortical loop.15Conclusion
The bi-tensor diffusion model can be used to detect the causes of microstructural changes in early PD, such as neurodegeneration and inflammation, more specifically. The model also provided more precise estimations of localized microstructural changes in early PD compared to the single-tensor model. Our findings demonstrate that neuroinflammation in early PD precedes the axonal degeneration and WM microstructural changes in PD precedes the changes of GM. Further, the bi-tensor model also can be used to show compensatory mechanisms in PD that occur in the cerebellum.Acknowledgements
The authors have no conflicts of interest to disclose. This work was supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) fromJapan Agency for Medical Research and development (AMED).References
- Cochrane CJ and Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology 2013;80:857-864.
- Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316-329.
- Oestreich LKL, Lyall AE, Pasternak O, et al. Characterizing white matter changes in chronic schizophrenia: A free-water imaging multi-site study. Schizophr Res 2017;189:153-161.
- Pasternak O, Sochen N, Gur Y, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009;62:717-730.
- Guttuso T, Jr., Bergsland N, Hagemeier J, et al. Substantia Nigra Free Water Increases Longitudinally in Parkinson Disease. AJNR Am J Neuroradiol 2018.
- Ofori E, Krismer F, Burciu RG, et al. Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Mov Disord 2017;32:1457-1464.
- Ofori E, Pasternak O, Planetta PJ, et al. Increased free water in the substantia nigra of Parkinson's disease: a single-site and multi-site study. Neurobiol Aging 2015;36:1097-1104.
- Ofori E, Pasternak O, Planetta PJ, et al. Longitudinal changes in free-water within the substantia nigra of Parkinson's disease. Brain 2015;138:2322-2331.
- Planetta PJ, Ofori E, Pasternak O, et al. Free-water imaging in Parkinson's disease and atypical parkinsonism. Brain 2016;139:495-508.
- Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 2012;32:17365-17372.
- Braak H and Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008;70:1916-1925.
- Wang Q, Liu Y and Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener 2015;4:19.
- Agosta F, Canu E, Stojkovic T, et al. The topography of brain damage at different stages of Parkinson's disease. Hum Brain Mapp 2013;34:2798-2807.
- Rektor I, Svatkova A, Vojtisek L, et al. White matter alterations in Parkinson's disease with normal cognition precede grey matter atrophy. PLoS One 2018;13:e0187939.
- Wu T and Hallett M. The cerebellum in Parkinson's disease. Brain 2013;136:696-709.
Figures
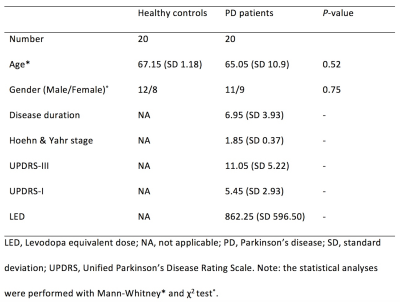
Table 1. Demographic characteristics of the participants.
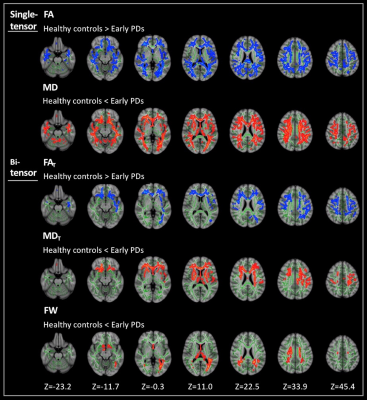
Figure 1. Comparison of single-tensor (FA and MD) and bi-tensor (FAT, MDT, and FW) between healthy controls and patients with early PD. TBSS analyses showed significantly (family-wise error corrected P < 0.05) lower FA and FAT (blue-light blue voxels) and significantly higher MD, MDT, and FW (red-yellow voxels) in patients with early PD compared with healthy controls. The skeleton is shown in green. To aid visualization, the results are thickened using the fill script implemented in FMRIB Software Library. FA, fractional anisotropy; FAT, free-water corrected FA; FW, free-water; MD, mean diffusivity; MDT, free-water corrected MD; TBSS, tract-based spatial statistics.
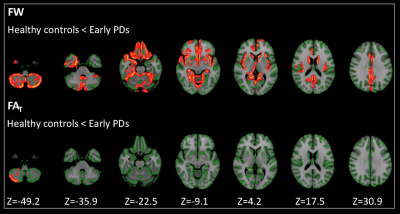
Figure
2. Comparison
of FAT and
FW between healthy controls and patients with early PD. GBSS analyses showed
significantly (family-wise error corrected P < 0.05) higher
FAT and
FW (red-yellow voxels) in patients with early PD when compared with healthy
controls. The skeleton is shown in green. To aid visualization, the results are
thickened using the fill script implemented in FMRIB Software Library. FAT, free-water
corrected FA; FW, free-water; GBSS, Gray matter-based spatial statistics."
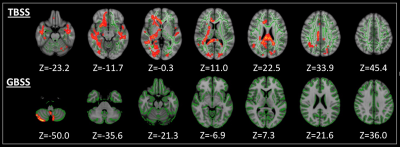
Figure 3. TBSS and GBSS analyses showed positive correlations (family-wise error corrected P < 0.05) between free-water and Unified Parkinson's Disease Rating Scale (UPDRS)-1, a non-motor severity scale, in the white and gray matter, respectively. The skeleton is shown in green. To aid visualization, the results are thickened using the fill script implemented in FMRIB Software Library. GBSS, Gray matter-based spatial statistics; TBSS, tract-based spatial statistics.