2306
A Pre-Clinical PET/MRI/MRS Study on Lactate Transport Inhibition by Bitter Melon Juice in Pancreatic Cancer Models1Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO, United States, 2Radiology, University of Colorado Denver, Aurora, CO, United States
Synopsis
Pancreatic cancer (PDAC) is a highly aggressive malignancy, displaying poor response to the frontline chemotherapeutics. PDAC cells undergo cellular reprograming to meet their bioenergetic and biosynthetic demands, with glycolytic shift emerging as the primary metabolic hallmark of carcinogenesis. Bitter melon juice (BMJ) is a widely consumed vegetable in Asia; recent studies have reported an increased AMPK phosphorylation and activation in BMJ-treated tumor xenografts. Here, we report on an inhibition of lactate export in PANC1 cells upon BMJ treatment, leading to acidification and cell death mediated by decreased transporter expression of GLUT1 and MCT4, both in vitro and in vivo.
Introduction:
Pancreatic cancer (including pancreatic ductal adenocarcinoma, PDAC) is the 4th leading cause of cancer death in the United States, with a 5-year survival rate of 4.8%-the lowest of any cancer sub-type. Recent times have witnessed a heightened interest in recognizing the potency of natural products as anticancer agents and their underlying mechanisms, particularly for PDAC. Bitter melon juice (BMJ) has been used in alternative medicine for decades against gout, hyperglycemia (type 2 diabetes), rheumatism 1. Recently, we have shown AMPK activation-mediated apoptotic cell death as a major mechanism in BMJ in in vitro and in vivo PDAC models 2. The goal of this study was to elucidate metabolic effects of BMJ in pancreatic cancer cell lines in vitro (1H/13C-MRS metabolomics on cell extracts) and in vivo (MRI/PET on mouse flank xenografts).Methods:
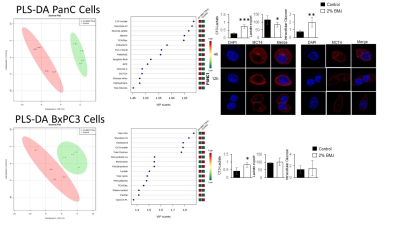
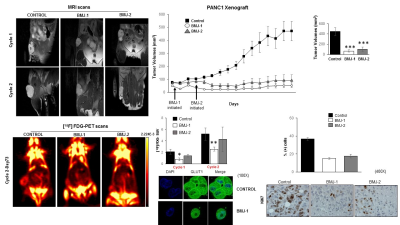
Two human PDAC cell lines, PANC1 and BxPC3 cell lines were treated with 2% BMJ (72 hr) with supplementing the culture media with 5mM [1-13C]glucose. All experiments were repeated as n=4. All 1H/13C-MRS on hydrophilic and lipophilic cell extracts and lyophilized media were obtained on a 400MHz Bruker Avance III spectrometer using a 5 mm-BBI probe, and multivariate analysis (3D-component partial least square linear discriminate analysis, PLS-LDA) was conducted with MetaboAnylast2.0 software. All animal studies were approved by the University of Colorado IACUC. Athymic female nude mice were inoculated with PANC1 cells into the dorsal right flank; the tumor growth was monitored by MRI and caliper measurements. Three study groups were designed (n=4 each): (i) early 200mg/kg BMJ-1 treatment (24 hrs post-inoculation); (ii) late 200 mg/kg BMJ-2 treatment (initiated when tumors reached 100 mm3); (iii) placebo group. All treatment was via oral gavage, 5 day a week. Anatomical proton-density (PD) weighted and diffusion weighted (DW) MRI was performed at a Bruker 4.7 Tesla MRI (with a 36-mm volume RF coil). RARE PD, to assess volume, parameters were as follows: FOV = 4cm; slice thickness = 1.2mm; TE/TR = 4000/31.9ms; number of slices = 16; number of averages = 2; matrix size = 180 degrees; total acquisition time = 4.1 minutes; b-values = 256x256. DWI parameters were as follows: FOV = 4.6cm; slice thickness = 2 mm; TE/TR = 3000/40ms; number of slices = 4; number of averages = 16; matrix size = 64x64; total acquisition time = 15 minutes; b-values = 0, 150, 300, 600, 800, 1000 s/mm2. 18F-Fluoroglucose (FDG, average 200 μCi) PET was acquired at a Siemens Inveon microPET scanner. All data analysis (tumor volumes, ADC and FDG-SUV) were performed using Bruker ParaVision (v4.0) and Siemens Inveon Research Workstation software. All MRS/MRI/PET data were correlated with immunohistochemistry/ immunofluorescence for Ki67 (proliferation), LDH, GLUT1 and MCT4 (lactate transporter).Results:
PLS-DA MRS metabolomics post 72 hrs of BMJ identified an almost complete inhibition of 13C-lactate transport from PANC1 (mutated KRAS) (P<0.05) but not in BxPC3 (wild-type KRAS) cells (Figure 1). Immunofluorescence studies on treated cells (in vitro) displayed a strong decrease MCT4 (lactate export). This was accompinied by a decrease glucose uptake into the cells. BMJ also induced cell death and reduced clonogenic potential of PDAC cells in vitro. In vivo, multi-modal imaging (MRI and FDG-PET) in PANC1 xenografts further reiterated the in vitro results, with BMJ-associated significant changes in tumor volumes, ADC and FDG-SUVs (Figure 2). The tumor growth was significantly decreased in both BMJ treated groups (P<0.002). ADC values, inversely correlating with the extent of tissue cellularity and the number of Ki67 cells, were shown to increase with BMJ treatment (from 1.05 x 10-3 mm/s to 1.56 x 10-3 mm/s). BMJ treatemnt significanthly decreased 18F-FDG glucose uptake into tumors, when compared to untreated PANC1 xenografts (P<0.01). Immunofluorescence studies on stained xenografts (ex vivo) displayed a strong decrease in GLUT1 (glucose uptake).Conclusions:
Our study provides an in-depth account of underlying mechanism of BMJ efficacy in modulating PDAC cell metabolism. We identify inhibited lactate export as a key target of BMJ efficacy in PDAC – this could provide a justification for initiating clinical trials for PDAC treatment with several novel compounds targeting the MCT4 transporter family.Acknowledgements
No acknowledgement found.References
[1] Dhar, D., et al. (2018) Mechanisms and Drug Targets for Pancreatic Cancer Chemoprevention. Curr Med Chem, 25, 2545-2565.
[2] Raina, K., et al. (2016) Promise of bitter melon (Momordica charantia) bioactives in cancer prevention and therapy. Semin Cancer Biol, 40-41, 116-129.