2090
Calcification and USPIO detection in Carotid Artery Plaque using QSM and SWI1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Cambridge University Hospitals NHS Foundation Trust, Cambridge Biomedical Campus, Cambridge, United Kingdom
Synopsis
In this work we demonstrated the feasibility and potential benefits of using Susceptibility Weighted Imaging and Quantitative Susceptibility Mapping for application in carotid artery plaque imaging for detection, quantification, and distinction of areas of calcification and USPIO uptake.
Introduction
Ultrasmall paramagnetic iron oxide (USPIO) particles offer a way to functionally assess carotid artery plaques by identifying areas of inflammation1. Increased susceptibility effects due to USPIO-uptake is associated with an increase in R2* and signal reduction on T2*w imaging. However, two acquisitions with a multi-contrast protocol and post-acquisition analysis are required to distinguish areas of paramagnetic USPIO-uptake (positive susceptibility) from areas containing other susceptibility sources such as diamagnetic calcification (negative susceptibility). Quantitative Susceptibility Mapping (QSM) directly measures the tissue magnetic susceptibility whilst susceptibility weighted imaging (SWI) generates contrast from the field it creates. This study investigates the use of QSM and SWI to identify and quantify USPIO uptake and distinguish it from calcification.Methods
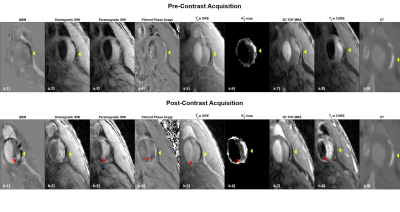
Seven patients with carotid artery disease were scanned at 1.5T (MR450w, GE Healthcare, Waukesha, WI). Two MRI examinations were performed per patient, before and 48 hours after 5mg/kg USPIO-injection (Ferumoxytol, AMAG Pharmaceuticals, Lexington MA). The study protocol comprised: 3D Time-of-Flight (TOF) MRA; black-blood, fat suppressed T1-w CUBE; 3D multi-echo gradient echo acquisition (SWAN); and a black-blood; fat suppressed, 3D multi-echo gradient echo acquisition (SWAN) (Figure 1). In all seven cases, the black-blood T2*-weighted SWAN sequence was used for SWI and in four cases the bright blood SWAN sequence was acquired and used for QSM processing, while the multi-contrast protocol was used for validation. QSM included IDEAL water-fat separation to estimate ΔB,2,3 background field removal, and dipole field inversion using MEDI4–11 (Figure 2a). SWI-processing consisted of generating a phase mask from the high-pass filtered phase of the first echo. This was subsequently multiplied four times with the magnitude data. Two different phase filters were used to selectively enhance T2*-effects caused by calcification or USPIO (Figure 2b)12. R2*-mapping was also performed. The images were manually co-registered by centring on the carotid bifurcation. USPIO and calcification were identified as areas of strongly positive and negative susceptibility values respectively using QSM. Each set of SWI showed darker regions in areas of either calcification or USPIO. On the high-pass filtered phase image calcification was identified by a positive phase shift and USPIO uptake by a negative one.
Areas of USPIO uptake, calcification, and normal tissue (susceptibility close to zero/minimal signal reduction on SWI) were outlined on QSM and SWI and the results were compared to the multi-contrast protocol. Calcification was identified as dark regions on all acquisitions with an increased R2*-value. In three cases CT was also available. USPIO-uptake was identified by comparing pre- and post-contrast images; uptake was bright on T1w images with increased R2* and darkening on T2*w images. For quantitative analysis, the signal intensities (SIs) on the T2*w magnitude images, relative to the sternocleidomastoid muscle, and the corresponding R2* and susceptibility values were determined for the different regions of interest.
Results
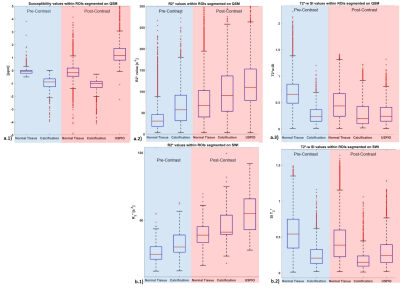
Areas of calcification appeared strongly diamagnetic on both pre- (μ=-0.98±0.45ppm) and post-contrast (μ=-1.18±0.53ppm) QSM, while USPIO uptake appeared strongly paramagnetic (μ=1.4±0.91ppm) on the second scan. A comparison of the SWI images showed improved visualisation of the calcification on the diamagnetic SWI, due to a positive value on the filtered phase, while the paramagnetic SWI demonstrated USPIO-uptake, due to a negative value on the filtered phase. All results were confirmed with the multi-contrast protocol and CT when available (Figure 3&4). In slices containing both calcification and USPIO-uptake, QSM and SWI processing allowed for a clear distinction between para- and diamagnetism (Figure 3&4). The quantitative analysis confirmed these results. Normal plaque tissue showed a relatively high SI on T2*-w images and low R2*. Regions containing calcification or USPIO, lead to reduced relative T2*w SIs and increased R2*. As expected, the SIs on T2*w images, R2* and QSM were relatively constant in calcified areas (Figure 5).Discussion
This study compared QSM and SWI for the identification of plaque calcification and USPIO uptake. Both QSM and SWI allowed for a much clearer delineation of the area of USPIO uptake compared to quadrant analysis, that is commonly used in evaluating USPIO uptake with T2*w sequences or R2*-mapping. Compared to T2*w images and R2*-maps, areas of calcification and USPIO uptake can be unambiguously identified and distinguished without a pre-contrast scan. The linear relationship between USPIO concentration and susceptibility value allows for the quantification of USPIO contrast agent uptake per voxel.Conclusion
Overall we have shown the use of QSM and SWI to identify USPIO uptake and unambiguously distinguish it from calcification. This aids the use of USPIOs as a quantitative biomarker of plaque inflammation.Acknowledgements
P Ruetten is funded by a Medical Research Council/Sackler Stipend. The project was supported by the Addenbrooke’s Charitable Trust and the NIHR comprehensive Biomedical Research Centre. A Usman is funded by Mountbatten Cambridge International Scholarship in collaboration with Cambridge Trust, Christ’s College and Sir Ernest Cassel Educational Trust.
We would like to thank Jianmin Yuan for his support with sequence development.
References
1. Gillard J, Graves M, Hatsukami T, Yuan C. Carotid Disease : The Role of Imaging in Diagnosis and Management. Vol. 9780521862264. Cambridge: Cambridge : Cambridge University Press; 2006.
2. Dimov A V., Liu T, Spincemaille P, Ecanow JS, Tan H, Edelman RR, et al. Joint estimation of chemical shift and quantitative susceptibility mapping (chemical QSM). Magn Reson Med. 2015;73(6):2100–10.
3. Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, et al. Multicoil Dixon chemical species separation with an iterative least‐squares estimation method. Magn Reson Med. 2004;51(1):35–45.
4. Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2013;69(2):467–76.
5. Kressler B, de Rochefort L, Liu T, Spincemaille P, Jiang Q, Wang Y. Nonlinear Regularization for Per Voxel Estimation of Magnetic Susceptibility Distributions From MRI Field Maps. IEEE Trans Med Imaging [Internet]. 2010;29(2):273–81. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=5067387%0Apapers2://publication/doi/10.1109/TMI.2009.2023787
6. De Rochefort L, Brown R, Prince MR, Wang Y. Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn Reson Med. 2008;60(4):1003–9.
7. Zhou D, Liu T, Spincemaille P, Wang Y. Background field removal by solving the Laplacian boundary value problem. NMR Biomed. 2014;27(3):312–9.
8. Liu J, Liu T, De Rochefort L, Ledoux J, Khalidov I, Chen W, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage [Internet]. 2012;59(3):2560–8. Available from: http://dx.doi.org/10.1016/j.neuroimage.2011.08.082
9. Liu T, Liu J, De Rochefort L, Spincemaille P, Khalidov I, Ledoux JR, et al. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: Comparison with COSMOS in human brain imaging. Magn Reson Med. 2011;66(3):777–83.
10. De Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: Validation and application to brain imaging. Magn Reson Med. 2010;63(1):194–206.
11. Shmueli K, Zwart J De. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 2009;62(6):1510–1.
12. Haacke EM, Xu Y, Cheng Y-CN, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52(3):612–8.
13 the codes referenced in [4-11] are available at "http://weill.cornell.edu/mri/pages/qsm.html"
Figures
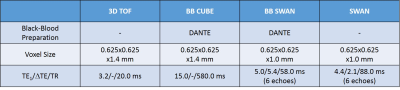
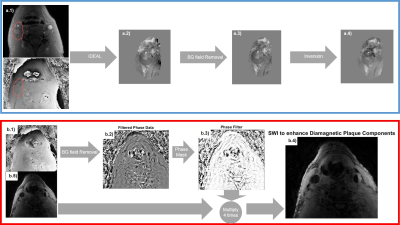
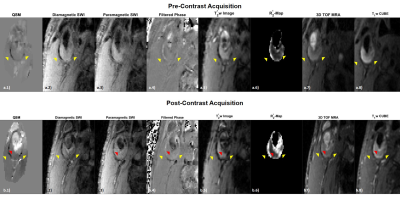
Figure2a): QSM consists of estimating ΔB(a.2), removing background fields(a.3), and dipole field inversion (a.4). Chemical shift artifacts in estimating ΔB(a.2), are corrected using IDEAL(a.2).
Figure2b): For SWI, the phase ϕ(b.1) is high-pass-filtered for background-field-removal(b.2). A phase mask M is generated to darken areas with positive phase shifts, e.g. calcifications(b.3): M={(π-ϕ)/π if ϕ≥0; 1;if ϕ<0}. Another mask can enhance visibility of paramagnetic materials, considering negative phase shifts: M(x,y)={(π+ϕ)/π if ϕ≤0;1if ϕ>0}. The masks are multiplied four times with the magnitude image(b.5) to improve visibility of calcification (b.4) or USPIO.