2078
QSM identifies calcification and intraplaque hemorrhage in patients with significant carotid stenosis1Weill Cornell Medicine, New York, NY, United States, 2Xuanwu Hospital, Beijing, China
Synopsis
We developed and evaluated carotid QSM for detecting calcification and intraplaque hemorrhage in carotid plaques. Preliminary results in patients with significant carotid stenosis showed QSM was able to detect both calcification and intraplaque hemorrhage in good agreement with findings on CT and multi-contrast MRI.
INTRODUCTION
Carotid plaques with intraplaque hemorrhage (IPH) are vulnerable to rupture and significantly increases stroke risk (1). Black blood multi-contrast MRI (2) can detect large IPHs with high accuracy but misses half of small or heavily calcified IPHs (3). Accurate discrimination of calcification from IPH is particularly important because unlike IPH, calcification may confer stability to large plaques. Quantitative susceptibility mapping (QSM) can reliably distinguish paramagnetic hemorrhage from diamagnetic calcification in the brain (4). However, QSM of carotid plaques remains challenging clinically due to artifacts caused by blood flow and presence of fat, bone, and air cavity in the neck region (5,6). Our objective was to develop robust QSM for calcification and IPH detection in carotid plaques and evaluate its performance in patients by comparing with CT and multi-contrast MRI.
METHODS
QSM acquisition. Multi-echo 3D GRE sequence was optimized for carotid QSM to achieve spatial resolution of 0.6x0.6x3 mm3 and 6 cm longitudinal coverage of the carotid bifurcation in approximately 5 min. Four echo times were acquired per TR with 4.7 ms echo spacing (corresponding to 4π inter-echo phase evolution of fat relative to water at 3T) which provides optimal field estimation in the presence of fat (7).
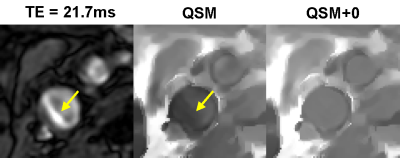
QSM+0 reconstruction. Effective background field removal in the neck is difficult due to the close proximity of blood vessels to bone and air with much stronger susceptibilities. Consequently, the total field inversion algorithm, which does not require separate background field removal, was used for field-to-source inversion (8). Furthermore, as the input field measurements in or near blood vessels is often corrupted by blood flow and residual chemical shift in fat (Fig.1), an additional constraint enforcing QSM uniformity within the vessel lumen (QSM+0) was introduced similarly to CSF regularization in the brain (9). This is a biophysically meaningful assumption as the arterial blood is well mixed in the heart and reaches the carotid bifurcation within 200 ms (10). QSM was computed by minimizing a nonlinear cost function using the Newton method with a preconditioned conjugate gradient solver in each Newton step:
$$y^*=argmin_y\frac{1}{2}{\parallel}w(f-d{\otimes}Py){\parallel}^2_2+{\lambda\parallel}M_G{\triangledown}Py{\parallel}_1+{\lambda_{A}\parallel}M_{A}P(y-\overline{y}_{A}){\parallel}^2_2$$
where $$$f$$$ is the input total field, $$$d$$$ is the magnetic dipole kernel, $$$P$$$ contains preconditioning weights (20 in the background air, 1 otherwise), $$$Py$$$ is the unknown susceptibility map, $$$w$$$ represents the noise weighting of the input field, $$$M_G$$$ and $$$M_A$$$ are the edge and artery lumen masks obtained from the magnitude image, respectively. Here the first term is the data fidelity term, the second term penalizes jumps in QSM map unless an edge is present, and the third term enforces smoothness within the arterial lumen.
Human carotid MRI. Five healthy volunteers and eight patients with significant carotid stenosis (>50% occlusion) were scanned on Siemens 3T scanners. Three patients had CT scans prior to MRI. The carotid MRI protocol consisted of TOF, 2D black blood multi-contrast sequences, and QSM. The vessel lumen masks were obtained using a custom semi-automated region-growing algorithm. The quality of QSM maps were scored on a 3-point scale (1=failed, 2=moderate, 3=excellent).
RESULTS
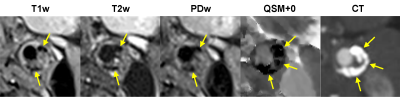
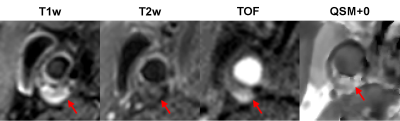
Figure 1 shows an example of artifact on the GRE magnitude image caused by the complex blood flow pattern in the carotid bifurcation, which causes artifact in QSM. This artifact was removed by QSM+0, leading to improved depiction of the artery. Figure 2 shows an example of the appearance of a carotid plaque with extensive calcification on multi-contrast MRI (hypointense), QSM (approximately -1.5 ppm with respect muscle, which is consistent with susceptibility of calcification (11)), and CT (hyperintense), demonstrating good visual agreement. Figure 3 shows an example of a plaque with IPH which appears hyperintense on T1w and TOF and hypointense on T2w (2) in concordance with high positive susceptibility on QSM (0.55 ppm, indicating hemorrhage). Overall QSM quality is 2.8 ± 0.4 in healthy volunteers and 2.3 ± 0.9 in patients (two patients had poor QSM due to excessive breathing motion). Six plaques with IPH and four with calcification were identified on multi-contrast MRI, all of which could be seen on QSM.DISCUSSION
Our initial patient results demonstrate that QSM+0 algorithm provides reliable detection of calcification and IPH in carotid plaques in good agreement with multi-contrast MRI and CT. Motion suppression techniques such as navigator will be essential for further improving the reliability of carotid QSM. QSM has the potential to improve carotid plaque characterization for treatment planning and stroke risk assessment.Acknowledgements
No acknowledgement found.References
1. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44(11):3071-3077.
2. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106(11):1368-1373.
3. Ota H, Yarnykh VL, Ferguson MS, Underhill HR, Demarco JK, Zhu DC, Oikawa M, Dong L, Zhao X, Collar A, Hatsukami TS, Yuan C. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology 2010;254(2):551-563.
4. Chen W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, Salustri C, Wang Y. Intracranial calcifications and hemorrhages: characterization with quantitative susceptibility mapping. Radiology 2014;270(2):496-505.
5. Wang C, Liu S, Chen Y, Buch S, Fan Z, Haacke EM, Yang Q. Intraplaque hemorrhage and calcification detection with quantitative susceptibility mapping. ISMRM 2018;491.
6. Ruetten P, Priest AN, Yuan J, Usman A, Gillard JH, Graves MJ. Phase corrected bipolar acquisition for simultaneous water-fat separation and quantitative susceptibility mapping of the carotid artery wall. ISMRM 2018;2194.
7. Pineda AR, Reeder SB, Wen Z, Pelc Cramér-Rao bounds for three-point decomposition of water and fat. Magn Reson Med 2005;54(3):625-635.
8. Liu Z, Kee Y, Zhou D, Wang Y, Spincemaille P. Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping. Magn Reson Med 2017;78(1):303-315.
9. Liu Z, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn Reson Med 2018;79(5):2795-2803.
10. Harloff A, Zech T, Wegent F, Strecker C, Weiller C, Markl M. Comparison of blood flow velocity quantification by 4D flow MR imaging with ultrasound at the carotid bifurcation. AJNR Am J Neuroradiol ;34(7):1407-1413.
11. Dimov AV, Liu Z, Spincemaille P, Prince MR, Du J, Wang Y. Bone quantitative susceptibility mapping using a chemical species-specific R2* signal model with ultrashort and conventional echo data. Magn Reson Med 2018;79(1):121-128.
Figures