2066
Highly accelerated NCE-MRA: Phase correction to remove background artefacts for complex subtraction1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Department of Radiology, Addenbrooke’s Hospital, Cambridge, United Kingdom
Synopsis
For subtractive Non-Contrast-Enhanced MR Angiography (NCE-MRA), complex subtraction of k-space data (prior to reconstruction) can exploit the sparsity of the difference images for under-sampled dataset reconstruction, but it causes background artefacts. This study proposed a phase correction method to restore the polarity of
Introduction
Subtractive Non-Contrast-Enhanced MR Angiography (NCE-MRA) methods, such as Fresh Blood Imaging (FBI)1, subtract images with different blood contrasts to produce background-suppressed angiograms. For acquisitions accelerated by compressed sensing and parallel imaging, complex subtraction of k-space data (prior to reconstruction) can exploit the sparsity of the subtracted data, achieving improved reconstruction quality at large acceleration factors2. However, the signal of some background tissues, such as muscle, appears higher on the dark-blood images. This signal should become negative after subtraction, but it will be shown as residual background tissue or artefacts due to the loss of its polarity in conventional magnitude reconstruction.
A phase correction method3 can be used to restore the polarity of negative signal and null background artefacts. This method requires a background reference for phase correction, which can be provided by the bright-blood dataset in subtractive NCE-MRA. This study aims to evaluate the background artefacts caused by complex subtraction in accelerated FBI, and then assess the performance of phase correction method in artefacts removal.
Method
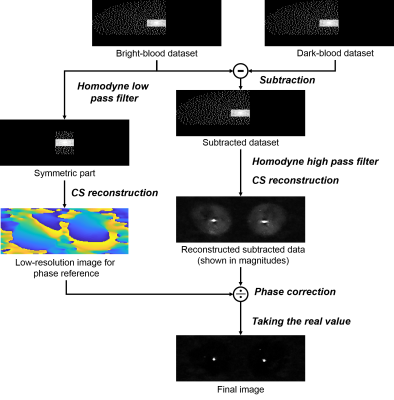
Fig. 1 shows the image reconstruction process. Complex subtraction of k-space data is performed prior to reconstruction. A low-resolution image is reconstructed from the symmetric central part of the bright-blood dataset and is used as the background reference to correct the phase of reconstructed subtracted data. Angiogram images with restored negative polarity can be then obtained by taking the real value instead of the absolute value of the corrected data. This step can be considered as the Homodyne phase-correction procedure for partial Fourier sampling, but it is also necessary for datasets without partial Fourier sampling.
In this study, undersampled data reconstruction was performed using the split-Bregman algorithm4 for compressed sensing reconstruction and SPIRiT5 for parallel imaging calibration. A Poisson-disk random under-sampling combined with partial Fourier in both ky (60%) and kz (75%) dimensions. The sample number is 10% of the total number of samples of the full k-space. Eight FBI datasets (TR 3 RR interval, TE 60ms, ETL 60–90, matrix 224×224, 80 slices of 2 mm, FOV 40 cm, acquisition time 1.5–2.5 minutes) were acquired from 10 healthy volunteers using a 1.5 T MRI system (450/450w, GE Healthcare, Waukesha, WI).
Background artefacts were evaluated using the ratio of background signal to arterial signal. The signal level was measured as the mean signal intensity within selected region of interest (ROI). The ROI for arterial signal was drawn manually to cover a representative segment of artery on the MIP, and the ROI for background signal was drawn on the background region where artefacts can be observed.
Results and discussion
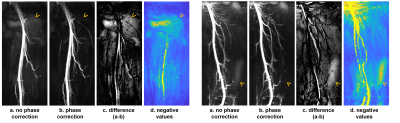
Fig. 2 shows max intensity projections (MIPs) of reconstruction results without (a) and with phase correction (b). Panel (c) shows the difference of the two MIPs, and panel (d) shows the magnitudes of negative values on the reconstructed images with restored polarity (d). Background artefacts can be observed on images without phase correction (a), coincided with the location of negative signal (d). This proves that the artefacts are caused by the negative signal which loses its polarity during reconstruction. These artefacts, although not strong, can obscure branch vessels with low intensity on the MIP (arrows). Phase correction removed these artefacts and generated a darker and clearer background (b).
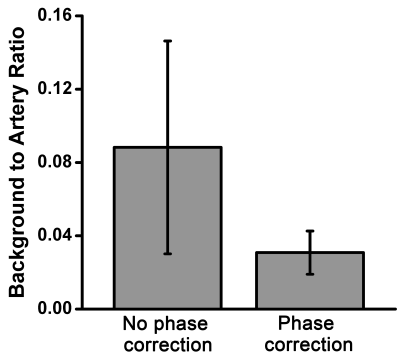
Quantitative analysis results are shown in Fig.3. By using the phase correction method, the ratio of background signal to arterial signal was reduced to 34.9% of the images without phase correction for FBI and FSD respectively. All of the differences were statistically significant (paired t-test, p <0.05).
Conclusion
The phase correction method using low-resolution bright-blood images as background reference can be used to restore the polarity of negative signal and remove background artefacts for highly accelerated subtractive NCE-MRA reconstruction with complex subtraction.Acknowledgements
The authors acknowledge the support of the Addenbrooke’s Charitable Trust and the NIHR Cambridge Biomedical Research Centre. Hao Li acknowledges the China Scholarship Council and Cambridge Trust for fellowship support. The authors thank Joshua Kaggie for providing the high-performance computer.References
1. Miyazaki M, Sugiura S, Tateishi F, Wada H, Kassai Y, Abe H: Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging 2000; 12:776–783.
2. Storey P, Otazo R, Lim RP, Kim S, Fleysher L, Oesingmann N, Lee VS, Sodickson DK. Exploiting sparsity to accelerate noncontrast MR angiography in the context of parallel imaging. Magn Reson Med. 2012;67(5):1391-1400.
3. Kellman P, Arai AE, Mcveigh ER, Aletras AH. Phase-Sensitive Inversion Recovery for Detecting Myocardial Infarction Using Gadolinium-Delayed Hyperenhancement. 2002;383:372-383.
4. Goldstein T, Osher S. The split Bregman method for L1-regularized problems. SIAM J Imaging Sci. 2009;2:323.5. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010;64(2):457-471.
5. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010;64(2):457-471.
Figures