1881
Octafluorocyclobutane as a novel 19F MRI gas agent for significant lung image quality improvement1Chemistry and Materials Science Program, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Chemistry, Lakehead University, Thunder Bay, ON, Canada, 4Biology, Lakehead University, Thunder Bay, ON, Canada, 5Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
Two different MRI gas techniques are presently being employed for lung imaging: hyperpolarized noble gas MRI and fluorinated inert gas MRI. Perfluoropropane (PFP) is the most widely used 19F gas for imaging purposes. In this work, we demonstrate the feasibility of using an octafluorocyclobutane (OFCB) as a novel gas contrast agent for 19F lung MRI. Furthermore, the direct comparison between OFCB and PFP was performed In Vivo and In Vitro. OFCB, due to its chemical structure and relaxation properties, demonstrated a higher SNR. Therefore, usage of OFCB can significantly improve the image quality of 19F lung MRI.
Introduction
Inert fluorinated gas MRI is a promising lung imaging modality, currently under development1. Perfluoropropane (PFP) is the most commonly used gas for fluorine-19 (19F) lung MRI. However, there is a signal-to-noise ratio limitation associated with a fast spin-spin relaxation2,3 and chemical shift artifact due to the molecular structure of PFP. The purpose of this study was to show the feasibility of using octafluorocyclobutane (OFCB) as a gas contrast agent for 19F lung MRI and to compare the performance of this gas with PFP images In Vitro and In Vivo. OFCB is non-toxic and contains eight chemically equivalent 19F nuclei per molecule as opposed to PFP which has only six. Furthermore, Friedrich et.al.4 reported that OFCB has an effective spin-spin relaxation constant (T2*) of 20ms which is much higher than PFP. These three facts make the OFCB promising candidate for the F lung MRI.Materials and Methods
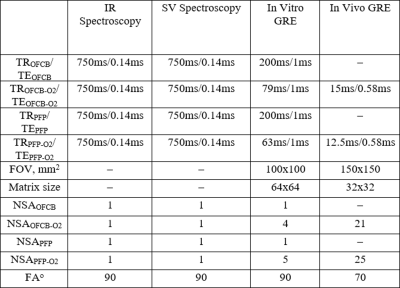
A clinical Philips Achieva 3T MRI scanner was equipped with custom-built quadrature coils tuned to the 19F resonance frequency. Four syringes filled with 8 ml of OFCB, PFP, a mixture of 21% of O2 and 79% of PFP, and a mixture of 79% of OFCB and 21% of O2 were used for the phantom study. T1 measurements were conducted using inversion recovery spectroscopy. The inversion time (TI) was varied in a range 4-48ms with a time step of 3ms for the pure gases and step of 1ms for OFCB-O2 gas mixture. The SNR dependence on TI was fitted using the T1 relaxation equation SNR=SNR0(1-2eTI/T1). The spectroscopy parameters are shown in Table 1. The bandwidth was equal to 32 kHz and the sampling number was equal to 2048 yielding the spectral resolution of 0.13 ppm.
Direct comparison of the single-voxel 19F spectra and gradient echo (GRE) images of the OFCB-O2 and PFP-O2 phantoms was conducted using the settings shown in Table 1. The T2* relaxation constant was acquired from the fit of free-induction decay (FID). The net GRE scan time was equal to 21s for both phantoms. Two healthy Spraque Dawley rats were imaged during breath-hold using the OFCB-O2 and PFP-O2 gas mixtures and the GRE pulse sequence with parameters shown in Table 1. The net scan time was equal to a breath-hold duration of 10s. The repetition time for the PFP-O2 scans was calculated using the T1 value published by Couch5. The animal experiments were conducted using an animal care protocol approved by the local animal care committee.
Results and Discussion
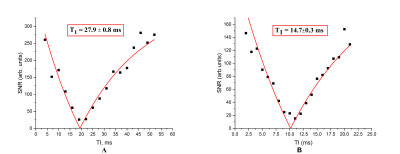
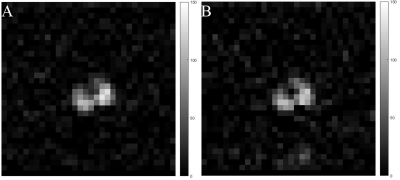
Fig.3 demonstrates the inversion recovery curves for the pure OFCB (A) and OFCB-O2 (B) gas mixture. The measured relaxation time constants are shown in Table 2. The OFCB-O2 gas mixture had 59% higher T2* and 18% higher T1 values compared to the PFP-O2 mixture. Fig.4A shows the spectra of the PFP-O2 mixture and the OFCB-O2 mixture. The SNR of the PFP-O2 and OFCB-O2 phantoms were equal to 362 and 500 respectively. The SNR of the OFCB-oxygen mixture was approximately 38% higher than the PFP-O2 SNR. Figures 4B and 4C illustrate two GRE images of the OFCB and PFP breathing mixtures. These images were acquired with unequal numbers of averages to keep the total scan time constant. The SNR was 52.72 for the PFP-O2 phantom and 58.82 for the OFCB-O2 phantom. In addition, due to the chemical structure of OFCB molecule, no chemical shift artifact was observed for this gas. Following phantom studies, In Vivo imaging of healthy rat lungs was conducted. Fig.5 shows the axial whole-lung projections obtained using OFCB-O2 gas mixture (Fig.5A) and PFP-O2 gas mixture (Fig.5B). The SNR of the OFCB image was equal to 10.6, whereas the SNR of the PFP image was equal to 8.73. Based on measured relaxation parameters, the theoretical SNR boost of OFCB was calculated and to be 19%. The measured SNRs of lung images agree with this theoretical value within error. The SNR advantage could be attributed to the higher number of chemically equivalent 19F atoms per OFCB molecule and a slower signal decay due to a larger T2* constant of OFCB compared to PFP. It should be noted, that even with a lower NSA value, the OFCB image had a 19% higher SNR value compared to the PFP. Furthermore, use of OFCB can decrease specific absorption rate (SAR).Conclusion
This work demonstrates the feasibility of using OFCB as a gas agent for 19F lung MRI. Using this fluorinated gas could significantly improve performance of 19F lung MRI due to its higher 19F signal, absence of chemical shift artifacts, and lower SAR.Acknowledgements
The authors would like to thank Lakehead University (LU) and the ThunderBay Regional Health Research Institute (TBRHRI) for partial support of this work and access to their facilities. Yurii Shepelytskyi is supported by Ontario Graduate Scholarship. Vira Grynko is supported by Ontario Trillium Scholarship. Francis Hane is supported by fellowships from the BrightFocus Foundation and the Canadian Institutes for Health Research.References
- Couch, M. J. et al. 19F MRI of the Lungs Using Inert Fluorinated Gases: Challenges and New Developments. J. Magn. Reson. Imaging (2018). doi:10.1002/jmri.26292
- Couch, M. J. et al. Pulmonary ultrashort echo time 19F MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: feasibility. Radiology.2013; 269:903–9.
- Kruger, S. J. et al. Functional imaging of the lungs with gas agents. JMRI. 2016; 43:295–315.
- Friedrich, J., Rivoire, J., Terekhov, M. & Schreiber, L. M. 19F-MRI: Flow Measurement of Fluorinated Gases During High Frequency Oscillatory Ventilation. in Proc. Intl. Soc. Mag. Reson. Med. 19
- Couch, M. J. et al. Inert fluorinated gas MRI: a new pulmonary imaging modality. NMR Biomed.2014; 27: 1525–1534.
Figures