1697
A comparative study of MR elastography and intravoxel incoherent motion based on volumetric analysis in the evaluation of histological grade of Hepatitis B virus-related hepatocellular carcinoma1Radiology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, 2Department of Radiology, Mayo Clinic, Rochester, Armenia, 3MR Research China, GE Healthcare, Beijing, China
Synopsis
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. Poorly differentiated HCC is associated with higher recurrence and worse survival compared with well and moderately differentiated HCC and preoperative prediction of histological grade is useful for deciding treatment strategy. We compared the value of MR elastography (MRE) and intravoxel incoherent motion (IVIM) in predicting the histological grade of hepatitis B virus (HBV)-related HCCs using volumetric analysis. Our results demonstrated that only mean tumor stiffness, and not ADC or IVIM metrics, could predict the histological grade of HCCs.
Introduction:
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer death worldwide, with increasing incidence and mortality [1][2]. Histological grade is one of the most important factors affecting patient prognosis [3, 4]. Therefore, preoperative diagnosis of histological grade could be helpful for the selection of treatment. MR Elastography (MRE) can be used to quantitatively assess the stiffness of tissue [5, 6]. Intravoxel incoherent motion (IVIM) is a functional MR imaging technique that can reflect the diffusion of water molecules and the microcirculation of tissue separately [7, 8]. Apparent diffusion coefficient (ADC) represents both pure diffusion and microcirculation, D (diffusion coefficient) is the diffusion parameter representing pure molecular diffusion, D* (pseudodiffusion coefficient) is the perfusion-related diffusion parameter representing incoherent microcirculation within the voxel, and f (perfusion fraction) is the fraction of the diffusion linked to microcirculation. Both techniques have shown potential value in distinguishing malignant liver tumors from benign tumors and predicting the histological grade of HCCs [9-13]. However, tumor heterogeneity cannot be totally reflected by mean values obtained from a region of interest (ROI) in one slice [14]. Volumetric analysis is a method which could capture the intratumoral heterogeneity [14, 15]. This study aimed to compare the performance of MRE and IVIM in predicting histological grade in hepatitis B virus (HBV)-related HCCs using volumetric analysis.Methods:
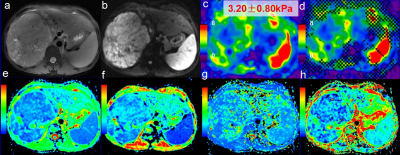
This retrospective study was approved by the institutional review board and written informed consent was waived. 70 patients (63 males and 7 females; age range, 22-72 years; mean, 49.2 years) were included in the study according to the following criteria: (a) pathologically confirmed HCCs after operation; (b) positive HBsAg in the serum or history of HBV infection; (c) no history of any previous radiotherapy, chemotherapy, and surgical treatment; (d) the time interval between the MR examination and surgery was one month or less; (e) no distinct motion artifacts or slice misregistration; and (f) the lesions were more than 2.5 cm in diameter. The patients were classified into poorly differentiated and well/moderately differentiated groups according to pathological results. All subjects underwent MRI exams using a 3.0T, whole-body, MRI scanner (Discovery MR750, GE Healthcare, Milwaukee, WI). A spin-echo (SE), flow-compensated, multislice, echo-planar imaging (EPI), 3D-MRE sequence using 60 Hz vibrations and respiratory-triggered axial diffusion-weighted imaging (DWI) using 11 b-values (b=0, 30, 50, 100, 150, 200, 300, 500, 800, 1000, 1500 sec/mm2) were performed. The MRE magnitude and phase images were processed using a direct-inversion (DI) algorithm to calculate the stiffness. Regions of interests (ROIs) were manually placed on each axial image of stiffness, ADC, D, D*, and f maps to encompass as much of the lesion as possible with reference to the T2W images. Afterwards, all ROIs were merged into a volume of interest (VOI) which covers the whole tumor using a manufacturer-developed tool (Omni-Kinetics, GE Healthcare). Mean values for the stiffness, ADC, D, D* and f of the whole tumor were calculated. The values of the poorly differentiated HCC and well and moderately differentiated HCC groups were compared with the Student t test or Mann–Whitney U test depending on the statistical distribution of the data.Results:
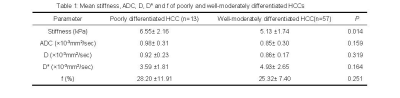
The number of poorly and well-moderately differentiated HCC was 13 and 57, respectively. The mean values for the stiffness, ADC, D, D* and f of the poorly and well-moderately differentiated HCCs is shown in Table 1. The mean stiffness of the poorly differentiated HCC group was significantly higher than that of the well-moderately differentiated HCC group (P= 0.014). On the other hand, the mean ADC, D, D* and f were not significantly different between the two groups (P= 0.159, 0.319, 0.164 and 0.25, respectively).Discussion:
By using volumetric analysis, the heterogeneity of the HCC may be captured better compared to single-slice analysis [14]. The mechanical properties of tissue are reflected by the stiffness and the diffusion and perfusion of the tumor is reflected by the ADC and IVIM metrics [5, 7]. Our study showed that the mean tumor stiffness has the potential to predict the histological grade of HCCs. However, the mean value for the ADC and IVIM metrics calculated over the whole tumor did not show usefulness for predicting HCC grade, which might be due to the influence of necrosis and hemorrhage in the volumetric analysis. Further development of these techniques is warranted to investigate the value of MRE and IVIM for predicting the histological grade of HCC.Conclusion:
Mean volumetric tumor stiffness may be more useful than the mean value of ADC and IVIM metrics for predicting the histological grade of HCCs preoperatively and thus might be useful for selecting more appropriate treatments and predicting prognosis.Acknowledgements
The authors state that this study has received funding by National Natural Science Foundation of China grant 81271562 (JW) and Science and Technology Program of Guangzhou, China 201704020016 (JW).References
[1] de Martel C, Maucort-Boulch D, Plummer M, et al. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology,2015,62:1190-1200.
[2] Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA CANCER J CLIN,2012,62:394-399.
[3] Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer,2002,95:1931-1937.
[4] Oishi K, Itamoto T, Amano H, et al. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol,2007,95:311-316.
[5] Glaser K J, Manduca A, Ehman R L. Review of MR elastography applications and recent developments. J Magn Reson Imaging,2012,36:757-774.
[6] Schwimmer J B, Behling C, Angeles J E, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology,2017,66:1474-1485.
[7] Iima M, Le Bihan D. Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology,2016,278:13-32.
[8] Le Bihan D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology,2013,268:318-322.
[9] Hennedige T P, Hallinan J T P D, Leung F P, et al. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. European Radiology,2016,26:398-406.
[10] Yoon J H, Lee J M, Yu M H, et al. Evaluation of hepatic focal lesions using diffusion-weighted MR imaging: Comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J Magn Reson Imaging,2014,39:276-285.
[11] Woo S, Lee J M, Yoon J H, et al. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology,2014,270:758-767.
[12] Thompson S M, Wang J, Chandan V S, et al. MR elastography of hepatocellular carcinoma: Correlation of tumor stiffness with histopathology features—Preliminary findings. Magnetic Resonance Imaging,2017,37:41-45.
[13] Shan Q, Chen J and Zhang T, et al. Evaluating histologic differentiation of hepatitis B virus-related hepatocellular carcinoma using intravoxel incoherent motion and AFP levels alone and in combination. Abdominal Radiology, 2017,42:2079-2088.
[14] Nougaret S, Vargas H A, Lakhman Y, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology,2016,280:446-454.
[15] Yoon S H, Park C M, Park S J, et al. Tumor heterogeneity in lung cancer: assessment with dynamic contrast-enhanced MR imaging. Radiology,2016,280:940-948.
Figures