1560
UTE and ZTE on an unmodified clinical whole-body MR system using an RF signal acquisition system operating in parallel achieving an acquisition delay of less then $$$2\,\mathrm{\text{µ}s}$$$1Experimental Cardiovascular Imaging Group, University Hospital of Ulm, Ulm, Germany, 2Research, Development and Technology (GBE), Sirona Dental Systems GmbH, Bensheim, Germany, 3Ulm University, Ulm, Germany
Synopsis
Ultra short echo time and zero echo time imaging on clinical systems are, even on modern systems, still limited by the rather long transient time required for switching from transmit to receive mode. In this contribution a complete receive-only chain was interfaced to an unmodified clinical whole-body MR system. Synchronization is achieved by a single trigger line as temporal reference and a time base signal, thus ensuring minimal interference and phase synchronous operation in parallel to the clinical system. An acquisition delay, between the real end of the excitation pulse and the beginning of signal acquisition, below $$$2\,\mathrm{\text{µ}s}$$$ could be realized.
Purpose
With continuously improving hardware ultrashort echo time (UTE) [1-3] and zero echo time (ZTE) [4-7] have gained increasing interest as techniques that enable new applications for magnetic resonance imaging (MRI). Key component for these techniques is rapid front end switching between transmit and receive mode thus enabling start of sampling with minimal latency and dead time. The latest generation of MRI systems has improved radio frequency (RF) front ends, however even these suffer from limited performance regarding dead time. Also there are many scanners of earlier generations deployed in the field. Due to their generic design, supporting many different coil settings and a large variety of applications, current clinical system face limitations in the achievable switching speed, thereby limiting the minimal acquisition delay, defined as the time between the real end of the excitation pulse and the beginning of signal acquisition, from the tens to hundreds of microseconds range on conventional clinical systems. In this contribution, we propose to interface a vendor independent receive-only system (guest) to a clinical MRI system (host). This approach needs no additional implementation for RF transmission or gradient control nor the programming of dedicated pre-scan procedures like shimming or RF power calibration, but allowing the optimization of front end switching. The guest is interfaced to the host by an trigger and a clock synchronization line. Customization of the host system is limited to programming a trigger output signal for guest/host synchronization, without the requirement for any mechanical or electrical modifications.Methods
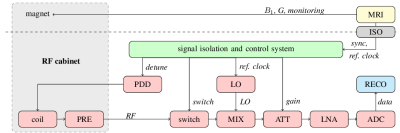
For proof-of-concept, a full receive chain based on standard industrial components has been designed and built (Fig. 1). The imaging sequence is executed on the host MR system. Spin excitation is performed by the in-built body coil, but the MR signal is being received by a independent receive chain operating in parallel. Synchronization of the guest system is done by an external trigger, generated by the host system, providing a temporal reference to the RF excitation pulse and a local oscillator clock reference. Both electrically isolated for better noise performance and safety considerations. The control of the guest is implemented in a field programmable gate array (FPGA), ensuring rapid and predictable response times. For MR signal reception, a dedicated single element coil with a short ring-down time has been designed [8]. The coil is actively detuned by a coil-optimized PIN-diode-driver (PDD). After passing an RF switch to improve signal isolation, the signal is down concerted by an analog mixer (MIX) to an intermediate frequency of $$$20\,\mathrm{MHz}$$$.To make use of the full dynamic range of the analog to digital converter (ADC) a configurable attenuator (ATT) is introduced followed by a low noise amplifier (LNA). The signal is sampled with $$$125\,\mathrm{MS/s}$$$ $$$(125\,\mathrm{MHz})$$$. Analog filters are replaced by digital post-processing and filtering. As all components of the receive chain are known, propagation delays can be compensated and the resulting latency is dominated by the dead time of the coil, which results in less than $$$2\,\mathrm{\text{µ}s}$$$.Results
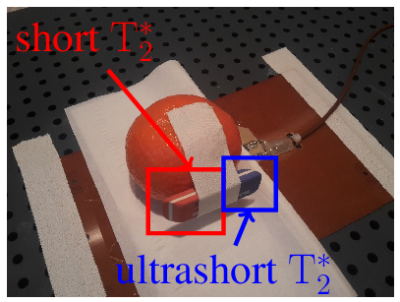
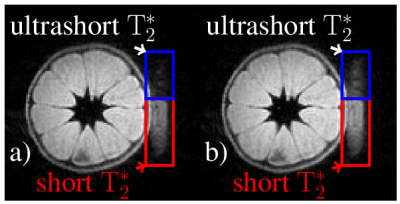
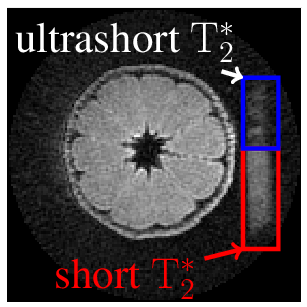
Data of a tangerine with attached eraser (Fig. 2) were acquired with 3D-UTE (Fig. 3, 4) and ZTE (Fig. 5). Both techniques used: field of view $$$\mathrm{FOV} = 100\,\mathrm{mm^3}$$$, isotropic spatial resolution $$$\Delta r = 1\,\mathrm{mm^3}$$$, flip angle $$$\mathrm{\alpha} = 3\,\mathrm{\text{°}}$$$, excitation pulse length $$$T_\mathrm{RF} = 25.6\,\mathrm{\text{µ}s}$$$ and were sampled in $$$32768$$$ equi-spaced center-out spokes. Maximal gradient strength was yielding a pixel bandwidth of $$$\mathrm{PBW} = 508.8\,\mathrm{Hz}$$$ at a repetition rate of $$$\mathrm{TR} = 7.7\,\mathrm{ms}$$$ for UTE and $$$\mathrm{PBW} = 524.5\,\mathrm{Hz}$$$ with $$$\mathrm{TR} = 4.400\,\mathrm{ms}$$$ for ZTE. The minimal acquisition delay of the clinical scanner was as $$$t_\mathrm{ad} = 136.2\,\mathrm{\text{µ}s}$$$, while for the proposed system a minimum of $$$t_\mathrm{ad} = 1\,\mathrm{\text{µ}s}$$$ could be realized. No apparent image artifacts can be observed in the $$$t_\mathrm{ad} = 1\,\mathrm{\text{µ}s}$$$ image, indicating sufficient data fidelity after tuning. A clearly improved appearance of the short $$$\mathrm{T}_\mathrm{2}^\mathrm{*}$$$ species can be appreciated. The acquired ZTE image demonstrates its feasibility on a standard clinical MR scanner.Conclusion
The proposed system is capable to interface to a clinical MR system. While this approach can intrinsically not overcome the limitations of procedures performed by the host system, like limited gradient strength or excitation pulse bandwidth, imaging with an acquisition delay below $$$2\,\mathrm{\text{µ}s}$$$ was realized. Where in our case, the method was used to show the feasibility to run UTE and ZTE, other important applications may be in the field of testing dedicated coils, not fitting to the standard coil interface of clinical scanners [8] or further investigation of advanced filter concepts as proposed by other groups [9-12].Acknowledgements
This project has received funding from the German research foundation under grant agreement RA 1960/9-1. The authors thank the Ulm University Center for Translational Imaging MoMAN for its support.References
[1] Brittain J., et al. Ultra-Short TE imaging with Single-Digit (8μs) TE, ISMRMR 11, (2004)
[2] Bracher A., et al. Feasibility of ultra-short echo time (UTE) magnetic resonance imaging for identification of carious lesions, MRM 66,(2011) 538 − 545.
[3] Robson M., et al. Magnetic Resonance: An Introduction to Ultrashort TE (UTE) Imaging, JCAT 27, (2003) 825 − 846.
[4] Hafner, S. Fast Imaging in Liquids and Solids with the Back-projection Low Angle ShoT (BLAST) Technique, MRI 12, (2012) 1047 − 1051.
[5] Madio, D., et al. Ultra-Fast Imaging Using Low Flip Angles and FIDs, MRM 34, (1995) 525 − 529.
[6] Weiger, M., et al. ZTE Imaging in Humans, MRM 70, (2013) 328 − 332.
[7] Weiger, M., et al. High-resolution ZTE imaging of human teeth, NMR in Biomedicine 25, (2011) 1144 − 1151.
[8] Horneff, A., et al. An EM simulation based design flow for custom-built MR coils incorporating signal and noise, IEEE TMI 37, (2017)527 − 535.
[9] Weiger, M., et al. Exploring the Bandwidth Limits of ZTE Imaging: Spatial Response, Out-of-Band Signals, and Noise Propagation, MRM74, (2015) 1236 − 1247.
[10] Kuethe D., et al. Transforming NMR Data Despite Missing Points, JMR 139, (1999) 18 − 25.
[11] Froidevaux R., et al. Filling the dead-time gap in zero echo time MRI: Principles compared, MRM 79, (2018) 2036 − 2045.
[12] Marjanovic J., et al. Multi-Rate Acquisition for Dead Time Reduction in Magnetic Resonance Receivers: Application to Imaging WithZero Echo Time, IEEE TMI 37, (2018) 408 − 416.
Figures