1088
The antibiotic doxycycline compromises cardiac mitochondrial and contractile function in diabetic mice1Preclinical & Translational MRI, Biomedical Engineering and Physics, Amsterdam UMC, Amsterdam, Netherlands, 2Lab Genetic Metabolic Diseases, Amsterdam UMC, Amsterdam, Netherlands
Synopsis
Given the evolutionary similarities between the bacterial and mitochondrial protein synthesis machinery, we tested the hypothesis that the widely used antibiotic doxycycline reduces mitochondrial function, and results in cardiac contractile dysfunction. Indeed, doxycycline exposure resulted in a dose-dependent reduction in maximal uncoupled mitochondrial respiration in cultured H9C2 cells. Maximal mitochondrial respiration was also reduced in mice treated with doxycycline. MRI of treated mice revealed contractile dysfunction as evidenced by marked diastolic and a mild systolic dysfunction. Moreover, doxycycline exacerbated mitochondrial and contractile dysfunction in animals with type 2 diabetes mellitus.
Introduction
In the heart, a high constant energy utilization rate is coupled to a constant energy production by OXPHOS in mitochondria [1,2], and as such, mitochondria serve a crucial role for optimal cardiac function. Because of the evolutionary similarities between the bacterial and mitochondrial protein synthesis machinery, certain classes of antibiotics, such as the widely used tetracyclines, also inhibit mitochondrial function [3], which in turn can affect cardiac contractile function. The diabetic heart is more vulnerable for contractile dysfunction because of mitochondrial dysfunction [4]. This might become even more apparent in combination with tetracycline antibiotics. In this study, we therefore aimed to investigate whether doxycycline impairs mitochondrial and contractile dysfunction in diabetes.Methods
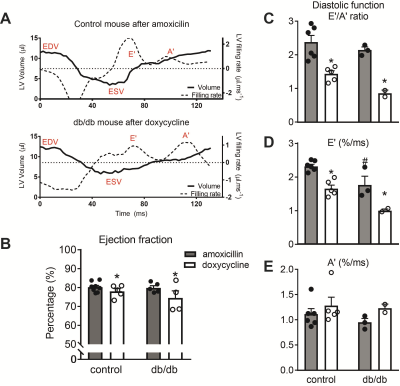
To establish the metabolic consequences of doxycycline, a tetracycline antibiotic, we tested the mitochondrial function in cultured H9C2 cells with (10 and 30 µg/ml) and without doxycycline treatment, by using an XFe96 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). To assess mitochondrial and contractile function in mice, drinking water of male non-diabetic (db/+) and diabetic (db/db) C57BL/Ks mice (10 weeks of age) was supplemented with doxycycline, or amoxicillin as a control antibiotic, for 2.5 weeks. Mitochondrial respiration was assessed by Oroboros O2k high-resolution respirometry [2]. Cardiac function in isoflurane-anaesthetized mice was assessed by cardiac MRI, using a 7.0 Tesla MR Solutions small animal scanner (MR Solutions, Guildford, UK) equipped with a 38-mm-diameter mouse volume coil. For left ventricle (LV) systolic function measurement, we used a cardio-respiratory gated multi-slice short-axis cine MRI acquisition using the following parameters: TR/TE = 7/2.8 ms, flip angle = 20°, FOV = 30×30 mm2, matrix size = 192×192, slice thickness = 1 mm, number of slices = 7, number of averages = 5, total acquisition time = 20 min. To assess diastolic function, a single mid-ventricular short-axis slice was acquired using a high frame rate retrospectively gated cardiac sequence [5] with the following sequence parameters: TR/TE = 7/2.35 ms, flip angle = 15°, FOV = 30×30 mm2, matrix size = 192×192, slice thickness: 1 mm, number of k-space repetitions = 400, acquisition time = 13 min. Off-line reconstruction of the diastolic function measurements was performed in MATLAB 8.1 (The Mathworks, Natick, MA, USA) using custom-built routines. In short, imaging data was binned into 60 frames and reconstructed by compressed sensing algorithms using Berkeley Advanced Reconstruction Toolbox (BART) [5, 6] (see Figure 3 for an example). All images were analyzed using MEDIS software (Leiden, The Netherlands). End-diastolic and end-systolic volumes of the left ventricle were used to calculate ejection fraction (EF) as a measure for systolic function. Diastolic function was measured as the ratio of the early or elastic (E’) and atrial (A’) filling rates.Results and Discussion
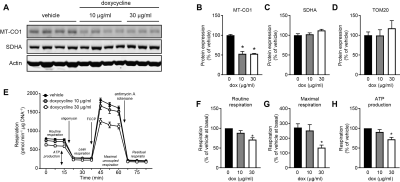
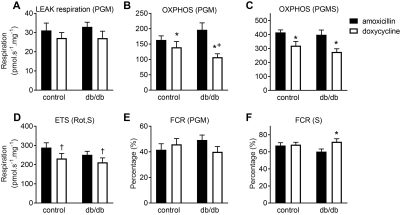
Doxycycline exposure resulted in a dose-dependent reduction in mitochondrially-encoded OXPHOS subunits with unaltered expression of nuclear-encoded subunits (Figure 1). Furthermore, doxycycline treatment showed lower basal mitochondrial and maximal uncoupled respiration in H9C2 cardiomyoblasts, which was also accompanied by a shift to glycolysis (data not shown). These data suggest that mitochondrial respiration was severely affected in H9C2 cardiomyoblasts treated with doxycycline, likely due to a disturbed balance between mitochondrial-encoded and nuclear-encoded mitochondrial subcomplexes. Maximal NADH (complex I)-linked mitochondrial respiration in the mouse heart was lower in doxycycline-treated control mice (p<0.001, Figure 2), and was more severely reduced in diabetic (db/db) mice treated with doxycycline (interaction effect: p=0.049). Maximal mitochondrial respiration was 26±4% lower in doxycycline-treated animals (p<0.001). A typical example of the self-gated retrospectively triggered mouse CINE MRI is given in Figure 3. Cardiac MRI confirmed that the untreated db/db animals suffered from heart failure with preserved ejection fraction (HFpEF), as the E’/A’ ratio was lower and EF similar compared to healthy controls. Cardiac MRI revealed a small, but significantly lower EF in both groups of doxycycline-treated animals. This reduction tended to be larger in db/db mice treated with doxycycline (Figure 4). Diastolic dysfunction in both groups treated with doxycycline was evident from the lower E’/A’ ratio compared to control (p=0.001). This reduction was solely due to lower filling rate during the E-phase (p<0.001).Conclusion
Doxycycline antibiotics reduce mitochondrial function in cardiac cells and mouse hearts in vivo. As a result, mice treated with doxycycline exhibited marked diastolic and mild systolic dysfunction. This negative effect of doxycycline on cardiac mitochondrial function was exacerbated in diabetic animals, resulting in a more severely affected cardiac contractile function.Acknowledgements
This work was in part supported by a grant from Amsterdam Cardiovascular Sciences and NWO Open Technologieprogramma MUSICIAN.References
1. Wüst RCI, Helmes M, Stienen GJM. Rapid changes in NADH and flavin autofluorescence in rat cardiac trabeculae reveal large mitochondrial complex II reserve capacity. J Physiol. 2015;593(8):1829-1840.
2.Wüst RCI, de Vries HJ, Wintjes LT, Rodenburg RJ, Niessen HW, Stienen GJ. Mitochondrial complex I dysfunction and altered NAD(P)H kinetics in rat myocardium in cardiac right ventricular hypertrophy and failure. Cardiovasc Res. 2016;111(4):362-372.
3. Moullan N, Mouchiroud L, Wang X, et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 2015;10(10):1681-1691.
4. Smith RL, Soeters MR, Wüst RCI, Houtkooper RH. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr Rev. 2018;39(4):489-517.
5. Motaal AG, Coolen BF, Abdurrachim D, et al. Accelerated high-frame-rate mouse heart cine-MRI using compressed sensing reconstruction. NMR Biomed. 2013;26(4):451-457.
6. Uecker M, Ong F, Tamir J, et al. Berkeley Advanced Reconstruction Toolbox. Proc Intl Soc Mag Reson Med. 2015;23:2486.
Figures