0996
In-vivo diffusion imaging of hippocampal network with 600 μm isotropic resolution at 7T1Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2School of Information Technology and Electrical Engineering, University of Queensland, Brisbane, Australia, 3Siemens Medical Solutions USA, Inc., Los Angeles, CA, United States, 4Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Neuroimaging findings indicate that neurological disorders differentially target distinct subregions of the hippocampal circuit 1. Therefore, the ability to image hippocampal network is essential to study the mechanism of the disease pathophysiology. Here we propose a novel framework that enables high-resolution intra-hippocampal macro-structural and network diffusion imaging.
Introduction
Given the relatively low spatial resolution of diffusion MRI, imaging intra-hippocampal network is challenging. Recently, 1mm isotropic diffusion MRI of the hippocampus was achieved 2 in a clinically feasible time frame by acquiring an axial slab around hippocampus and by utilizing a low b-value EPI strategy. Pushing the imaging resolution decreases the SNR and CNR, which could negatively affect the power to resolve intricate subfields of the hippocampus. Here we take advantage of 7T MRI technology, readout segmented diffusion MRI and a robust SNR enhancement technique, to enable diffusion MRI of the hippocampal network at 600 mm3isotropic resolution.Data acquisition
Readout segmented diffusion MRI sequence 3 was used to acquire 40 axial slices around hippocampus with isotropic resolution of 600 mm3, 12 diffusion-encoding gradient directions with one non-weighted diffusion image with b-value of 1000 and total scan time of 18 minutes. on a 7 Tesla whole-body scanner (Magnetom Terra, Siemens Healthcare, Erlangen, Germany) using a single-channel quadrature transmit radiofrequency (RF) coil, and a 32-channel receive array coil (Nova Medical Inc., MA), we used 11 readout segments, TR=7800ms, TE(b0)=77, TE=129, flip angle=170-degree, and acceleration factor=3. The institutional review board of the University of Southern California approved the study.Data analysis
In order to enhance SNR without a substantial sacrifice in spatial resolution and to denoise the magnitude images, we used a variation of the method described in Refs 4,5. This approach takes advantage of the high-level of structural correlation that is observed between different diffusion-weighted images, and unlike many nonlinear denoising approaches, the trade-off between spatial resolution and SNR can be rigorously characterized theoretically 4. This theoretical characterization confirms that substantial SNR improvement is possible with minimal loss of spatial resolution 4, allows automatic parameter tuning to optimize SNR-resolution efficiency 4, and also helps to ensure that important spatially-localized diffusion behavior will be preserved after denoising (unlike many other diffusion denoising schemes, which can encounter difficulties in the presence of rare/subtle diffusion features that are only present in a small number of voxels 5). Unlike some previous works 4–6, we did not have image phase information or information about the spatially-varying noise distribution, so our denoising approach only reduced the variance associated with noise without compensating for the noise floor (bias) associated with magnitude images 6.
Tractography was performed using Quantitative Imaging Toolkit (QIT) 7. Deterministic tensor-based tractography was performed within a region of interest that was drawn around the hippocampus. We used registered high-resolution T2w images to accurately pinpoint a spherical seed in a multilayer region of the hippocampus. The T2w was acquired with in-plane resolution of 300 μm2 and slice thickness of 2 mm.
Result
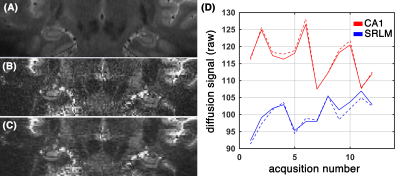
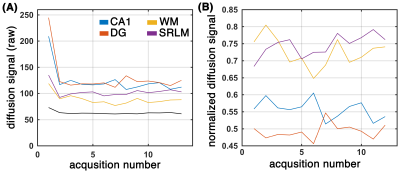
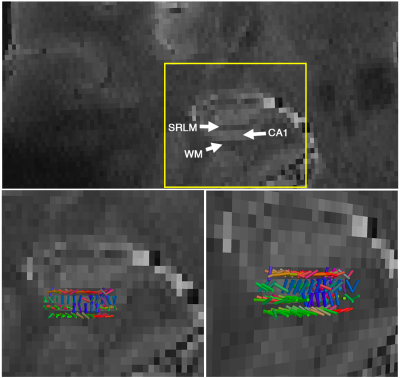
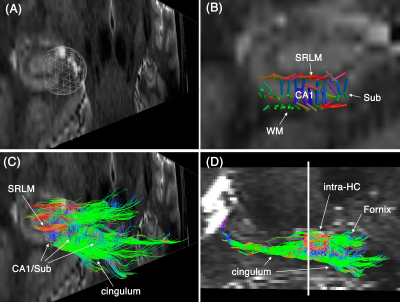
Subfields of the hippocampal formation were resolved using the proposed framework (Figure 1.b-c). The SNR enhancement technique improved the diffusion MRI quality (Figure 1c), without over-smoothing the data (Figure 1.d shows that the diffusion-weighted signal in gradient encoding domain was preserved). Diffusion-weighted signal was higher than the noise level (Figure 2.a) and contained orientational information (Figure 2.b) across subregions of the hippocampus. The expected fiber architecture of the CA1, startum radiatum and collateral white matter regions were captured (Figure 3). In addition, the expected along-hippocampal and intra-hippocampal connectivity were extracted and corroborate neuroanatomical topology (Figure 4).Conclusion
We were able to perform diffusion imaging of hippocampal network with 600 μm3 resolution, by taking advantage of higher signal in ultra-high field and by combining a high-resolution diffusion imaging sequence 3 and a post-processing diffusion signal enhancement technique 4,5. This resolution is 4.6 times higher than the state-of-the-art diffusion imaging at 1 mm3.Acknowledgements
This work was supported by NIH grants: 2P41EB015922-21, 1P01AG052350-01 and USC ADRC 5P50AG005142.References
1. Small, S.A., Schobel, S.A., Buxton, R.B., Witter, M.P. & Barnes, C.A. Nat. Rev. Neurosci.12, 585–601 (2011).
2. Treit, S., Steve, T., Gross, D.W. & Beaulieu, C. Neuroimage182, 479–487 (2018).
3. Porter, D.A. & Heidemann, R.M. Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med.62, 468–475 (2009).
4. Haldar, J.P. et al. Magn. Reson. Med.69, 277–289 (2012).
5. Kim, J.H., Song, S.-K. & Haldar, J.P. Magn. Reson. Med.75, 852–858 (2015).
6. Varadarajan, D. & Haldar, J.P. IEEE Trans. Med. Imaging34, 2191–2202 (2015).
7. Cabeen, R.P., Laidlaw, D.H. & Toga, A.W. ISMRM-ESMRMB Abstr.12–14 (2014).
Figures