0815
Rapid protocol for T1 and T2* mapping: STrategically Acquired Gradient Echo (STAGE) at 1.5 T MRI1IRCCS Fondazione Don Carlo Gnocchi, Milano, Italy, 2Radiology, Wayne State University, Detroit, MI, United States, 3The MRI Institute for Biomedical Research, Bingham Farms, MI, United States, 4Department of Electronics, Information and Bioengineering, Politecnico di Milano, Milan, Italy
Synopsis
Multi-contrast quantitative mapping has become an important research direction for characterizing brain tissue in physiological and pathological conditions. A rapid quantitative mapping acquisition is fundamental for its application in clinical routine. In this study, we adapted the STrategically Acquired Gradient Echo (STAGE) protocol for a clinical 1.5T MRI scanner. Using STAGE, we reconstructed longitudinal and effective transverse relaxation time maps. We obtained values similar to those in the literature in different brain structures with high repeatability. These results suggest that STAGE can be used at 1.5T and provide a variety of quantitative maps with an acquisition time of 7 minutes.
Introduction
Quantitative mapping of longitudinal (T1), transverse and effective transverse (T2*) magnetic resonance (MR) relaxation times, proton spin density (PSD), and quantitative susceptibility mapping (QSM) are useful for characterizing brain tissue1 in physiological and pathological conditions1-8.
Usually, sequences for mapping T1, T2*, PSD and QSM are acquired separately8-10, with long scanning time making translation into clinical practice difficult. The STrategically Acquired Gradient Echo (STAGE) method11,12 provides T1- and PSD-weighted (T1W, PSDW) images; susceptibility weighted imaging (SWI); T1, PSD, and R2*(1/T2*) maps and QSM in 5 minutes at 3T and 7 minutes at 1.5T13. The STAGE sequences and quantitative maps generation were recently described11,12 using 3 T scanners. This study describes a STAGE protocol for a 1.5 T scanner in order to 1) investigate if obtained T1 and T2* are similar to values reported by literature; 2) assess repeatability.
Methods
Subjects: 22 healthy subjects (HS, 36.4±19.3 years, 16 females). One HS (35 years, female), scanned on 10 different days.
MRI protocol: The scans were performed on a 1.5 T Siemens Magnetom Avanto scanner at IRCCS Fondazione Don Carlo Gnocchi ONLUS (Milan, Italy), using a 12-channel head coil. The STAGE protocol consisted of two double-echo gradient echo (GRE) SWI scans acquired with full flow compensation on each echo, with a total scan time of 7 minutes. Parameters, published at 3T11,12, were adapted for 1.5 T (Table 1) as follows. We simulated the gray matter (GM)/white matter(WM) contrast, the T1 precision, and the GM SNR as a function of Flip Angle (FA), in order to identify two FAs that allowed to reach a good trade-off among the parameters.
Quantitative maps: Data were processed with an in-house Matlab (MathWorks) program11,12. The T1, PSD, radiofrequency (RF) transmit, and RF receiver field maps were obtained from the two first echoes and the transmit and receiver field inhomogeneities were corrected11. The SWI was generated from the second echo with the lower FA. An R2* (1/T2*) map was obtained averaging the R2* maps obtained from the two echoes of each scan.
Relaxation times extraction from tissues of interest: Non-brain tissue was removed from the T1W, using the Brain Extraction Toolbox14. Deep GM structures (Caudate Nucleus –CN; Putamen –Put; Thalamus –Thal; Globus Pallidus –GP) were segmented on T1W using FIRST toolbox15. The masks were eroded to exclude border regions.The CSF, WM, GM partial volume maps were obtained using FAST toolbox16 and thresholded to 0.95 to exclude the voxels affected by partial volume effect. The Substantia Nigra (SN) and the Red Nucleus (RN) were manually segmented on SWI images on the axial slice where the structures were prominent and the borders were well defined. The median T1 and R2* were computed within each structure mask. The coefficient of variation (COV) was computed for each measure, for the 22 subjects and for the same subject acquired 10 times separately.
Results
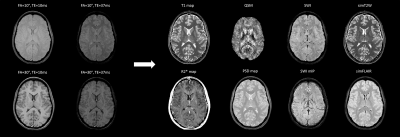
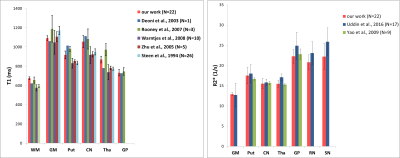
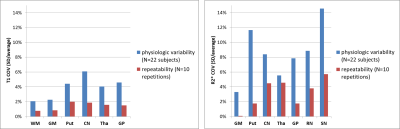
All the maps were reconstructed in about 5 minutes for each subject. The four acquired magnitudes and the reconstructed maps and qualitative contrasts are shown in Figure 1. The T1 and R2* of the different tissues are represented as bars in Figure 2 (our results and published values17-21). Figure 3 shows the T1 and R2* COV for the different tissues.Discussion
We adapted the STAGE sequences for a 1.5 T scanner. In order to provide all the maps displayed in Figure 1, different sequences are usually added in a research setting. Decreasing the acquisition times is a strong requirement for translation into clinical setting. The strategic combination of multi-TE and multi-FA makes it possible to obtain all the maps by acquiring only two gradient echo SWI images in 7 minutes.
The T1 values in the segmented structures obtained using the STAGE protocol have a good consistency among subjects (Figure 3): the intersubject COV is between 2% and 8%, similarly to the physiologic variability seen in17-19,21. The physiologic variability of R2* was higher, similarly to other work22. T1 and R2* repeatability was good: the COVs ranged from about 0 to less than 6%, similarly to the ones recently reported for a phantom23. The semiautomatic segmentation increased reproducibility with respect to manual drawing.
Our T1 and R2* results are similar to those reported in previous studies using a 1.5 T scanner17-22 (Figure 2), that used sequences specifically designed for obtaining T1 mapping.
Acknowledgements
We thank Siemens Healthineers for providing us the IDEA software.References
(1) Cercignani M, Dowell NG, Tofts PS. Quantitative MRI of the brain: Principles of physical measurement. CRC Press; 2018. (2) Chen Y, Liu S, Buch S, Hu J, Kang Y, Haacke EM. An interleaved sequence for simultaneous magnetic resonance angiography (MRA), susceptibility weighted imaging (SWI) and quantitative susceptibility mapping (QSM). . 2018. (3) Liu M, Liu S, Ghassaban K, et al. Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. J Magn Reson Imaging. 2016;44(1):59-71. (4) Liu S, Buch S, Chen Y, et al. Susceptibility-weighted imaging: Current status and future directions. NMR Biomed. 2017;30(4):10.1002/nbm.3552. Epub 2016 May 18. (5) Ghassaban K, Liu S, Jiang C, Haacke EM. Quantifying iron content in magnetic resonance imaging. Neuroimage. 2018. (6) Nürnberger L, Gracien R, Hok P, et al. Longitudinal changes of cortical microstructure in parkinson's disease assessed with T1 relaxometry. NeuroImage: Clinical. 2017;13:405-414. (7) Gracien RM, Jurcoane A, Wagner M, et al. Multimodal quantitative MRI assessment of cortical damage in relapsing-remitting multiple sclerosis. J Magn Reson Imaging. 2016;44(6):1600-1607. (8) Granziera C, Daducci A, Donati A, et al. A multi-contrast MRI study of microstructural brain damage in patients with mild cognitive impairment. NeuroImage: Clinical. 2015;8:631-639. (9) Baudrexel S, Nürnberger L, Rüb U, et al. Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early parkinson's disease. Neuroimage. 2010;51(2):512-520. (10) Costa-Mallen P, Gatenby C, Friend S, et al. Brain iron concentrations in regions of interest and relation with serum iron levels in parkinson disease. J Neurol Sci. 2017;378:38-44. (11) Wang Y, Chen Y, Wu D, et al. STrategically acquired gradient echo (STAGE) imaging, part II: Correcting for RF inhomogeneities in estimating T1 and proton density. Magn Reson Imaging. 2018;46:140-150. (12) Chen Y, Liu S, Wang Y, Kang Y, Haacke EM. STrategically acquired gradient echo (STAGE) imaging, part I: Creating enhanced T1 contrast and standardized susceptibility weighted imaging and quantitative susceptibility mapping. . 2018;46(2/23/2018):130-139. (13) Wang Y, Huang F, Xu W, et al. STAGE imaging at 1.5T: A rapid brain protocol providing more images as well as quantitative data. Intl Soc Mag Reson Med. 2018;26:2750. (14) Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-155. (15) Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907-922. (16) Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45-57. (17) Rooney WD, Johnson G, Li X, et al. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2007;57:308-18. (18) Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson Med. 2008;60:320-9. (19) Zhu DC, Penn RD. Full-brain T1 mapping through inversion recovery fast spin echo imaging with time-efficient slice ordering. Magn Reson Med. 2005;54(3):725-731. (20) Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003;49(3):515-526. (21) Steen RG, Gronemeyer SA, Kingsley PB, Reddick WE, Langston JS, Taylor JS. Precise and accurate measurement of proton T1 in human brain in vivo: Validation and preliminary clinical application. Journal of Magnetic Resonance Imaging. 1994;4(5):681-691. (22) Uddin MN, Lebel RM, Wilman AH. Value of transverse relaxometry difference methods for iron in human brain. Magn Reson Imaging. 2016;34(1):51-59. (23) Jiang Y, Ma D, Keenan KE, Stupic KF, Gulani V, Griswold MA. Repeatability of magnetic resonance fingerprinting T1 and T2 estimates assessed using the ISMRM/NIST MRI system phantom. Magnetic resonance in medicine. 2017;78(4):1452-1457.Figures