0694
Single-step Quantitative Susceptibility Mapping Reconstruction without Brain Extraction using Deep LearningHongjiang Wei1, Steven Cao1, Fuhua Yan2, Kristen W Yeom3, and Chunlei Liu1
1University of California, Berkeley, Berkeley, CA, United States, 2Rui Jin Hospital, Shanghai Jiao Tong University, Shanghai, China, 3Department of Radiology, Stanford University, Palo Alto, CA, United States
Synopsis
We propose a deep learning-based single-step QSM reconstruction method that directly estimates magnetic susceptibility from total phase images without brain extraction. 209 healthy subjects with ages ranging from 11 to 82 years were employed for network training. The trained network provides superior image quality and high reconstruction speed compared to other single-step QSM methods. In addition, the elimination of the brain extraction step makes it possible to estimate susceptibility near the boundaries and explore the magnetic susceptibility of whole brain vascular images.
Introduction
Quantitative susceptibility mapping (QSM) reconstruction requires several processing steps including phase unwrapping, background phase removal and dipole inversion1. Unfortunately, most background phase removals require edge erosion of the brain tissue resulting in invalid results near the edges. In addition, skull striping methods fail to define accurate neonatal brain regions and in vivo mouse brain, resulting in artifacts in the computed susceptibility images. To address these challenges, we propose a learning-based QSM reconstruction method that directly estimates the magnetic susceptibility from total phase images without brain extraction, referred to as autoQSM. Our results demonstrate that autoQSM has superior image quality and high reconstruction speed compared to other single-step QSM methods.Methods
209 healthy subjects with ages ranging from 11 to 82 years old were included for training. The subjects were scanned using a 3T GE MR750 scanner with the following parameters: FOV=22×22 cm2, matrix size=256×256, FA=20°, TR=54.6ms, TE1/spacing/TE8=5.46/3/26.5 ms, spatial resolution=0.86×0.86×1 mm3. Our network architecture is modified from an established U-net2. The overall network architecture used in this study is summarized in Fig.1. The normalized 3D total phase images were used as the input of the neural network and STAR-QSM3 images were used as the label. Out of the 209 healthy datasets, 42 subjects were used as a validation set. The proposed network structure was implemented using Python and Tensorflow using NVIDIA 1080TI GPU. To test the network’s ability to reconstruct QSM directly from total phase images, we applied the trained network to an infant brain data (2 years old), patients with Multiple Sclerosis (MS) and hematoma, and an in vivo mouse brain data (87μm isotropic resolution); all are dissimilar to the training data.Results
Fig.2 shows the three orthogonal views of total phase and QSM images on one healthy subject using five QSM reconstruction methods. Least Normal QSM (LN-QSM)4 exhibits some residual artifacts near the cortical regions. SS-TGV-QSM5 showed the significant artifact reduction but it also had substantially reduced susceptibility contrast between the cortical gray and white matter. AutoQSM preserves the susceptibility contrast similarly to COSMOS and STAR-QSM. In addition, autoQSM reveals cortex and cortical veins which were otherwise inaccessible using other methods as pointed by red arrows in Fig.2b. The autoQSM method was applied to the infant, child and adult subjects, patients with MS and hematoma which were not used for training dataset. The predicted results by autoQSM revealed comparable contrasts to those of STAR-QSM. From the difference maps (Fig.3c), there are negligible susceptibility differences related to the gray and white matter. The clear differences at the edge of the brain were caused by blood vessels that were predicted by the trained neural network. Fig.4d illustrates autoQSM’s remarkable capability of reconstructing QSM from total phase on an in vivo mouse brain. Yellow arrows pointed to amygdala regions with shadowing artifacts that are significantly reduced using autoQSM. Red arrows point to brain erosion that can be recovered using autoQSM. The white matter in the medulla can be well inviable as pointed by red arrow in Fig.4d, which is completely inaccessible on STAR-QSM images.Discussion
In this study, we constructed a deep neural network that performs QSM reconstruction from total phase images without brain volume extraction. One significant advantage is the high computational efficiency of the trained neural network achieved by combining two techniques: (i) skipping brain mask extraction processing, and (ii) end-to-end QSM processing computed by GPU. Compared to conventional QSM reconstruction methods and other single-step methods, our results show better QSM image quality. In addition, the training data used in this study covers a large age range (11 to 82 years old), which is important for high reproducibility in a longitudinal study. The preliminary results tested on infant brain data, patients with hematoma and mouse brain suggest that autoQSM can be applied to brain data dissimilar to the training data, which reveals that the network has generalized the underlying principles of QSM inversion.Conclusion
Our results demonstrate a powerful new paradigm for QSM reconstruction without brain volume extraction, which is implemented with a deep neural network that learns the underlying mathematical relationship between the total field and the magnetic susceptibility. Our results demonstrate that autoQSM has superior image quality compared to other single-step QSM methods.Acknowledgements
This work is supported by NIMH 096979 and NIH BRAIN Initiative U01 EB025162.References
1. Liu, C., et al., Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging, 2015. 42(1): p. 23-41. 2. Ronneberger, O., P. Fischer, and T. Brox. U-net: Convolutional networks for biomedical image segmentation. in International Conference on Medical image computing and computer-assisted intervention. 2015. Springer. 3. Wei, H., et al., Streaking artifact reduction for quantitative susceptibility mapping of sources with large dynamic range. NMR Biomed, 2015. 28(10): p. 1294-303. 4. Sun, H., et al., Whole head quantitative susceptibility mapping using a least-norm direct dipole inversion method. Neuroimage, 2018. 179: p. 166-175. 5. Chatnuntawech, I., et al., Single-step quantitative susceptibility mapping with variational penalties. NMR Biomed, 2017. 30(4).Figures
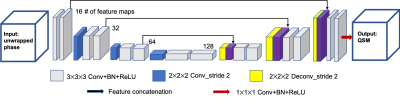
Figure 1. The schematic diagram of the neural network structure of autoQSM. A 3D U-net was implemented with 14 convolutional layers with a kernel size of 3×3×3, 1 convolutional layer with a kernel size of 1×1×1, 3 convolutional layers with a kernel size of 2×2×2 applied with stride 2, 3 deconvolutional layers with a kernel size of 2×2×2 applied with stride 2, and 3 feature concatenations.
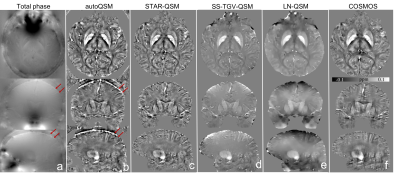
Figure 2. Comparison of different QSM reconstruction methods in a healthy subject. Red arrows point to vessels can be visible at the cortical surface which are recovered by autoQSM.
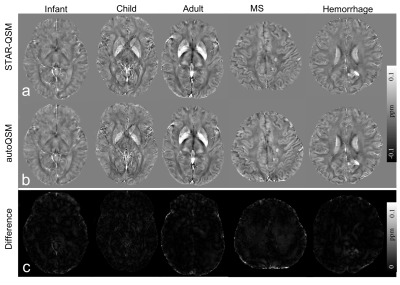
Figure 3. Comparison of QSM images computed using STAR-QSM and autoQSM methods. The last row shows the absolute difference maps between QSM images reconstructed by the two methods.
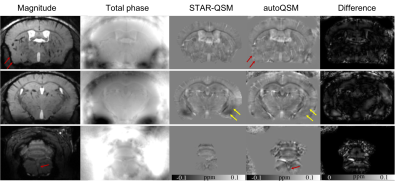
Figure 4. Representative axial slices of QSM images computed using STAR-QSM and autoQSM methods in an in vivo mouse brain. (a) magnitude images, (b) total phase maps, (c) QSM image reconstructed using STAR-QSM, (d) QSM images predicted using the trained neural network. Red arrows point to the brain edge loss caused by brain extraction on the QSM images. Yellow arrows point to reduced artifacts by the trained neural network.