0491
Glycogen detection in human brain via natural abundance 13C MRS at 7T1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3Philips Healthcare, Gainesville, FL, United States, 4Pediatrics, UT Southwestern Medical Center, Dallas, TX, United States, 5Chemistry, University of Texas at Dallas, Dallas, TX, United States, 6Internal Medicine, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Large quantities of abnormally-branched brain glycogen are hypothesized to be accumulating in disorders such as Lafora disease and Adult Polyglucosan Body Disease (APBD). However, non-invasive tools for brain glycogen detection in vivo are lacking. In this work we have used natural abundance 13C MRS at 7T with NOE, to detect glycogen in both normal and APBD brain. Qualitative comparison of the respective glycogen C1 signals in these two cases indicates no dramatic increase of the detectable glycogen concentration in APBD. To our knowledge this is the first human cerebral glycogen detection via natural abundance 13C MRS.
Purpose
Brain glycogen is an important source of energy, not only in cases of pathophysiological glucose insufficiency such as during transient reduced perfusion, but also in the course of normal brain activity. Abnormalities in glycogen metabolism and its storage have been implicated in various diseases such as Lafora disease and Adult Polyglucosan Body Disease (APBD). It is hypothesized that large quantities of abnormally-branched brain glycogen that cannot be metabolized are accumulating in these conditions1. However, quantitation of glycogen in brains of patients with this disorder has not been reported. Quantitative measurements of brain glycogen are thus desirable for diagnosis and treatment follow up. However, non-invasive tools for brain glycogen detection in vivo are lacking. Normal brain glycogen in mice is 100% 13C NMR visible2, suggesting that excess brain glycogen could be detected. Glycogen concentration in the normal brain is low and natural abundance 13C glycogen has not been detected in the human brain. Recent 13C infusion studies3,4 have produced estimates of brain glycogen concentration in the range of 3-10 mmol/g. However, the prolonged infusion protocols (2 days) required for human studies would be difficult for patients to tolerate. In this work we have optimized natural abundance 13C MRS, capitalizing on the increased sensitivity of high magnetic fields (7T) and NOE signal enhancement, to allow glycogen detection in normal controls. We have also applied the method in a case of an APBD patient. To our knowledge this is the first direct measurement of human cerebral glycogen via natural abundance 13C MRS.Methods
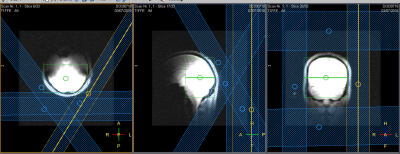
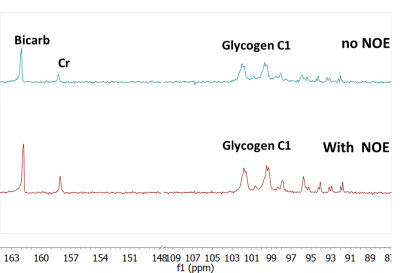
All procedures were performed with local IRB approval and after obtaining a written consent. In adult volunteers (controls=2; APBD patient=1), proton-coupled 13C NMR spectra were acquired on a whole-body 7T scanner (Achieva, Phillips Healthcare) with the subject laying comfortably supine, with a quadrature 1H/13C transmit/receive partial volume coil positioned below the occipital region of the head. Saturation slabs were utilized to minimize signal contribution from the occipital belly of the epicranial and trapezius muscles (Figure 1). The 13C spectra were acquired for approximately one hour (NSA = 8k), with TR = 400 ms, BW 16 kHz and 4k data points, frequency offset centered on the glycogen C1 signal at 100 ppm. To increase 13C SNR, NOE enhancement was implemented with B1 = 10 μT, 5% duty cycle, 100 ms mix time centered at 180 Hz downfield of water. The NOE enhancements were initially confirmed in a glycogen phantom containing 100 mM glycogen, 40 mM creatine, and 25 mM 100% 13C enriched bicarbonate (a reference).Results and Discussion
In
the phantom experiments, a clear NOE enhancement of glycogen C1 SNR was observed
at about 50%, Figure 2. The in vivo acquisition
protocol, while comparatively long, was well tolerated. In the normal control
brain, no glycogen C1 signal was detected when NOE was not utilized (data not
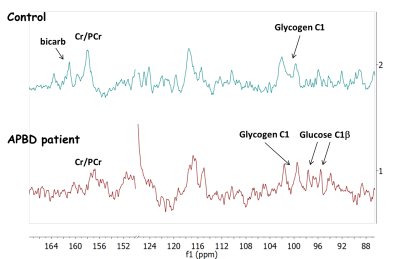
shown). With NOE, glycogen C1 doublet (at approximately 100 ppm) was observed in
both the normal controls and the APBD patient (Figure 3). Qualitative
comparison of the respective SNR in these two cases indicates no dramatic increase
of the detectable glycogen concentration in APBD. Given the reported several-fold
increase of glycogen in these patients and the poor solubility of the abnormal
glycogen, it is possible that the NMR visibility is compromised in this disease,
in contrast to previous findings on normal brain glycogen2. Lastly,
in the control case, if we assume a bicarbonate concentration of 25mM and
bicarbonate T1=10s, a rough estimate of glycogen concentration of
about 5mM can be obtained, consistent with previous 13C infusion
findings.Conclusion
We demonstrated the feasibility of detection of brain glycogen via natural abundance 7T 13C MRS with NOE. While the SNR of the detected signals is low and does not currently allow for reliable quantification of the observed spectra, the sensitivity of this method could be improved in the future by implementing closely-fitting 13C receive arrays, as well as with the availability of homogeneous volume excitation transmit coils. Assuming that the glycogen content of the brain of a patient with APBD is increased, these results suggest that 13C NMR visibility of abnormal glycogen or similar branched-chain structures is reduced in APBD.Acknowledgements
This work was supported in part by grants from the National Institutes of Health (EB-015908 and HL-034557) and Cancer Prevention Research Institute of Texas (RP150456), and an internal pilot award (FY19-IA0001).References
1. Raben N, Danon M, Lu N, Lee E, Shliselfeld L, Skurat AV, Roach PJ, Lawrence JC Jr, Musumeci O, Shanske S, DiMauro S, Plotz P. “Surprises of genetic engineering: a possible model of polyglucosan body disease”. Neurology 56 (12): 1739-45, 2001.
2. Lei H, Morgenthaler F, Yue T, Gruetter R. “Direct validation of in vivo localized 13C MRS measurements of brain glycogen”. Magn Reson Med. 57(2):243-8, 2007.
3. Öz G, DiNuzzo M, Kumar A, Moheet A, Seaquist ER. “Revisiting Glycogen Content in the Human Brain”. Neurochem Res. 40(12):2473-81, 2015.
4. Soares AF, Gruetter R, Lei H. “Technical and experimental features of Magnetic Resonance Spectroscopy of brain glycogen metabolism”. Anal Biochem. 529:117-126, 2017.
Figures