0294
Evolution of cerebral hemodynamics and metabolism across the early lifespan in patients with sickle cell disease1Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 2Pediatric Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Sickle cell disease (SCD) is an inherited hemolytic anemia with altered hemodynamics and increased stroke risk. However, lifetime trends in SCD cerebral hemodynamics and metabolism are poorly understood. We used non-invasive functional measures of cerebral blood flow (CBF) from arterial spin labeling, and oxygen extraction fraction (OEF) from T2-Relaxation-Under-Spin-Tagging MRI, to quantify hemo-metabolic patterns in controls (n=64) and SCD patients (n=125) across the early lifespan (6-40 years). CBF decreases with age in healthy controls (-3.2 mL/100g/min per decade) but increases in patients (5.2 mL/100g/min per decade). OEF was elevated in SCD, showing a similar slope with age as controls.
Introduction
Sickle cell disease (SCD) is an inherited hemolytic anemia in which sickled erythrocytes cause reduced oxygen carrying capacity, elevated cerebral blood flow (CBF) and oxygen extraction fraction (OEF), and increased stroke risk (1-4). Children with SCD receive transcranial Doppler ultrasound, and if velocities are significantly elevated, receive regular blood transfusions for primary stroke prevention for one year followed by transition to hydroxyurea (5). However, no mechanism for primary stroke prevention in adults with SCD exists, and adult patients typically only receive regular transfusions for secondary stroke prevention.
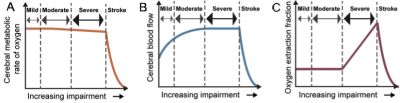
In SCD, as oxygen carrying capacity reduces, CBF will increase; when insufficient, OEF may elevate (Figure 1). However, it is unclear whether CBF reduces with age in adults with SCD, as has been observed in controls (6), or whether CBF increases with age to maintain OEF. MRI is a promising technology to evaluate these differences, relative to 15O-PET, as MRI can now measure CBF and OEF without exogenous contrast agents or ionizing radiation. Here, we use pseudo-continuous arterial spin labeling (pCASL) (7) and T2-relaxation-under-spin-tagging (TRUST) (8) to measure CBF and OEF, respectively, in individuals with SCD and controls across an early SCD lifespan.
Methods
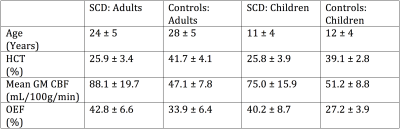
Study participants. Adults (n=69; age=24±5 years) and children (n=56; age= 11±4) with SCD, and race-matched adults (n=47; age= 28±5 years) and children (n=17; age=12±4) without sickle trait or history of stroke provided informed, written consent, and were scanned at 3.0T (Philips; Table 1).
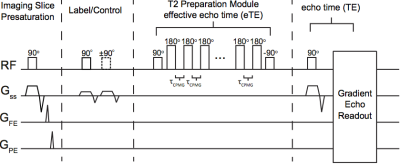
Experiment. CBF measurements were acquired with pCASL (Adults: PLD=1900; TR/TE=3675/13; Children: PLD= 1650; TR/TE=4000/16.7). PLD was optimized for children and adults separately based on known differences in blood arrival times between populations (7). OEF measurements were acquired with TRUST (τCPMG=10ms; eTE=0, 40, 80, 160; TR/TE=1978/3.6 ms, averages=3; Figure 2). Hematocrit (Hct) measurements were acquired via venipuncture within 7 days of MRI.
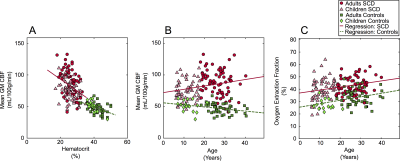
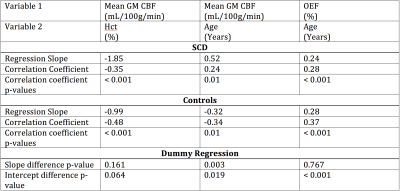
Analysis. Whole brain gray matter CBF was calculated from pCASL data using a standard kinetic model (9). TRUST data were pair-wise subtracted, and a single-exponential was fit to superior sagittal sinus signal as a function of TE. R2 was calculated and converted to venous oxygenation (Yv) using measured Hct, difference signal, and an established blood calibration model (10). OEF was calculated as OEF=(Ya-Yv)/Ya where Ya was determined from pulse oximetry and Yv from the above TRUST procedure. To investigate hemo-metabolic evolution with age, regression analyses were performed for (i) mean gray matter CBF versus age, and (ii) mean OEF versus age (Figure 3). Dummy variables were used to test if regression slopes were different between SCD and control groups (Table 2).
Results
CBF was observed to decrease with age in healthy controls by 3.2 mL/100g/min per decade of life, consistent with literature (6). However, CBF was found to increase with age in SCD patients by 5.2 mL/100g/min per decade of life (Figure 3; p=0.003). Regression results are summarized in Table 2. Additionally, although the upward trend in OEF with age is nearly identical between healthy controls and SCD patients (p=0.767), OEF was elevated throughout lifespan in SCD patients, with the intercept difference = 11.4% (p<0.001).Discussion
This study demonstrates that CBF increases with age in SCD, but decreases in healthy controls. Additionally, an upward trend in OEF with age is similar between controls and SCD patients, but OEF is elevated at all stages of life in SCD. These findings suggest that while CBF decreases moderately with age in healthy controls, even in late childhood and early adulthood, in SCD there is a moderate CBF increase with age. This is likely caused by upregulation of CBF to compensate for declines in oxygen carrying capacity, secondary to a combination of (i) changes in oxygen binding efficiency (which could arise from mild pulmonary dysfunction with age), (ii) hematocrit and red blood cell deformability reductions (11), and (iii) in some patients, increases in marovascular arterial steno-occlusion. This compensatory pattern of increasing CBF in SCD may be adequate in many patients, as OEF increases with age at a nearly identical rate in controls and SCD patients. These values should be useful references for how cerebral hemodynamics vary with age in patients with SCD, and could be useful for identifying dysfunction that may predispose patients for new or recurrent infarct.Conclusion
Using regression analysis of CBF and OEF metrics from non-invasive MRI, we showed that OEF is elevated uniformly in SCD patients throughout life compared to controls. Additionally, CBF decreases throughout life in healthy controls, but increases in patients with SCD.Acknowledgements
No acknowledgement found.References
1. Debaun MR, Derdeyn CP, McKinstry RC, 3rd. Etiology of strokes in children with sickle cell anemia. Ment Retard Dev Disabil Res Rev 2006;12(3):192-199.
2. Seakins M, Gibbs WN, Milner PF, Bertles JF. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest 1973;52(2):422-432.
3. Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Annu Rev Med 2013;64:451-466.
4. Arkuszewski M, Krejza J, Chen R, Melhem ER. Sickle cell anemia: reference values of cerebral blood flow determined by continuous arterial spin labeling MRI. Neuroradiol J 2013;26(2):191-200.
5. Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet 2016;387(10019):661-670.
6. Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011;21(6):1426-1434.
7. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73(1):102-116.
8. Lu H, Ge Y. Quantitative evaluation of oxygenation in venous vessels using T2-Relaxation-Under-Spin-Tagging MRI. Magn Reson Med 2008;60(2):357-363.
9. Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med 2002;48(2):242-254.
10. Lu H, Xu F, Grgac K, Liu P, Qin Q, van Zijl P. Calibration and validation of TRUST MRI for the estimation of cerebral blood oxygenation. Magn Reson Med 2012;67(1):42-49.
11. Renoux C, Romana M, Joly P, et al. Effect of Age on Blood Rheology in Sickle Cell Anaemia and Sickle Cell Haemoglobin C Disease: A Cross-Sectional Study. PLoS One 2016;11(6):e0158182.
Figures