0183
Mediators of the real-time fMRI amygdala neurofeedback training effect on PTSD symptom reduction: a whole brain structural equation model mapping analysis1Laureate Institute for Brain Research, Tulsa, OK, United States, 2Neuroscience Department, George Mason University, Fairfax, VA, United States, 3Department of Psychological Science, University of Arkansas, Fayetteville, AR, United States, 4Stephenson School of Biomedical Engineering, University of Oklahoma, Norman, OK, United States
Synopsis
We determined mediators of the real-time fMRI neurofeedback (rtfMRI-nf) amygdala training inducing PTSD symptom reduction in combat veterans. Thirty-six veterans with PTSD (25 experimental group and 11 control group receiving a feedback from a control region) completed three rtfMRI-nf training sessions in separate days. We employed a novel whole brain structural equation model mapping (SEMM) analysis to identify brain regions that mediated the effects of the rtfMRI-nf procedure on PTSD symptoms. Results revealed that PTSD symptom reduction in the experimental group was mediated by lower activation than the control group in default mode network regions during the neurofeedback training.
Purpose
Therapeutic use of real-time functional magnetic resonance imaging neurofeedback (rtfMRI-nf) has been investigated for treating psychiatric disorders. Although the potential of rtfMRI-nf as a treatment technique for psychiatric disorders has been demonstrated1, the neural underpinnings of symptom relief due to the training are insufficiently understood from the whole brain perspective. The present study aimed to identify brain activations that may function as mediators or moderators of the association between rtfMRI-nf and PTSD symptom change for combat veterans with PTSD. For this purpose, we devised a novel whole-brain structural equation model mapping (SEMM) analysis.Methods
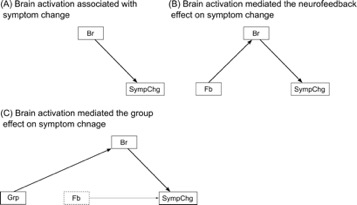
Thirty-six veterans with PTSD completed three rtfMRI-nf training sessions across three separate days. Training was designed to increase a neurofeedback signal either from the left amygdala (experimental group [EG], n=25) or from a control region not implicated in emotion regulation (control group [CG], n=11) during positive autobiographical memory recall2. PTSD Checklist-Military Version (PCL-M)3 was used to measure symptom levels one week prior to and one week after the training sessions. Brain activation that mediated or moderated the effect of neurofeedback training on symptom change was mapped voxel-wise in the whole brain with the structural equation model (SEM mapping: SEMM). The analysis was performed with sem package in R4. Figure 1 shows the path diagram of the model. The model assumed that the feedback signal (Fb) affected brain activation in a given region (Fb->Br) and an activation of this region was associated with symptom change after the training (Br->SympChg). The model also included the effect of the EG/CG group (Grp: EG=0, CG=1) on the feedback signal (Grp->Fb). The direct paths from feedback to symptom change (Fb->SympChg) and from the group factor to a brain activation (Fb->Br) were also included, considering an unidentified mediating factor could exist. In addition, moderation paths (dotted arrows) from the group factor onto the Fb->Br path and from the brain activation onto the Fb->SympChg path were included in the model. The moderation factors were modeled with an interaction of groups by feedback signal (Gr_x_Fb) and an interaction of feedback signal by brain activation (Fb_x_Br). Symptom change was the difference between the pre- and the post-training sessions (SympChg=post–pre), with a regression that pulled out the effect of baseline symptom score. The brain activation and feedback signals were an average of training runs of all sessions.Results
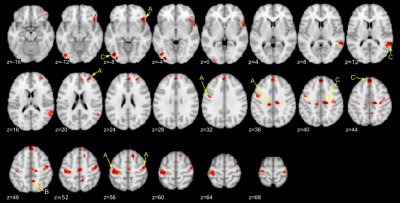
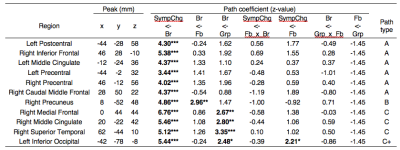
We focused on paths linking brain activation and symptom change, Br->SympChg and Fb_x_Br->SympChg, respectively, to explore the mediating and moderating roles of brain activation for a therapeutic effect on neurofeedback training. Figure 2 shows the regions with a significant coefficient at Br->SympChg path. No significant region was found for Fb_x_Br->SympChg path. Table 1 shows peak coordinates of regions with significant Br->SympChg path coefficients obtained from SEM analysis. The SEM analysis in each peak location revealed three types of significant path structures, which are depicted in Figure 3.
Type A is a region associated with symptom change without the effect of a feedback signal and group assignment (the left postcentral, the bilateral precentral, the right inferior frontal, the left middle cingulate, and the right anterior frontal regions). These regions were positively correlated with symptom change, indicating that the less activation in these regions, the greater the symptom decrease. The regions that mediated the feedback effect on symptom change (type B) were found in the right precuneus. Activation in the right precuneus was positively correlated with both a feedback signal and PCL-M change. The regions that mediated the group effect on symptom change (type C) were found in the right medial frontal, the right middle cingulate, the right superior temporal, and the left inferior occipital regions. A positive path coefficient in the Gr->Br means that a region’s activation was lower for the experimental group than for the control group.
Discussion
Precuneus activation was positively correlated with both the feedback signal and symptom change. This is interesting because a high feedback signal was associated with low symptom reduction, and since the precuneus activity was associated with negative emotion processing in PTSD5,6, this association might reflect the intrusion of negative valence in increasing the feedback signal that could lessen symptom reduction.
The brain activations mediating the group effect on symptom change were seen in the medial frontal area, the cingulate cortex, and the superior temporal area. These regions are part of the default mode network (DMN)7,8. A correlation between high DMN activity and the low perception of an emotional signal was demonstrated for healthy participants9, suggesting that the increased DMN activity could reflect a difficulty in positive emotion processing.
Acknowledgements
This research was supported by W81XWH-12-1-0697 award from the U.S. Department of Defense, the Laureate Institute for Brain Research (LIBR), and the William K. Warren Foundation.References
1. Young, K.D., G.J. Siegle, V. Zotev, et al., Randomized Clinical Trial of Real-Time fMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall. Am J Psychiatry, 2017;174(8):748-755.
2. Zotev, V., R. Phillips, M. Misaki, et al., Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. Neuroimage Clin, 2018;19:106-121.
3. Weathers, F.W., B.T. Litz, D.S. Herman, et al. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. in Annual Convention of the International Society for Traumatic Stress Studies. 1993. San Antonio, TX.
4. Fox, J., Z. Nie, and J. Byrnes, sem: Structural Equation Models. R package version 3.1-9, 2017.
5. Etkin, A. and T.D. Wager, Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry, 2007;164(10):1476-88.
6. Cwik, J.C., G. Sartory, M. Nuyken, et al., Posterior and prefrontal contributions to the development posttraumatic stress disorder symptom severity: an fMRI study of symptom provocation in acute stress disorder. European Archives of Psychiatry and Clinical Neuroscience, 2016:1-11.
7. Raichle, M.E., A.M. MacLeod, A.Z. Snyder, et al., A default mode of brain function. Proc Natl Acad Sci U S A, 2001;98(2):676-682.
8. Buckner, R.L., J.R. Andrews-Hanna, and D.L. Schacter, The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci, 2008;1124:1-38.
9. Wiebking, C., M. de Greck, N.W. Duncan, et al., Are emotions associated with activity during rest or interoception? An exploratory fMRI study in healthy subjects. Neuroscience Letters, 2011;491(1):87-92.
Figures