0083
Traumatic Brain Injury fast-forwards Alzheimer's parthenogenesis: A radiological-pathological investigation in a mouse AD model.1The Queensland Brain Institute, The University of Queensland, Brisbane, Australia
Synopsis
Traumatic brain injury (TBI) today is the strongest epigenetic risk factor for Alzheimer's disease (AD) . To date, the underlying mechanisms of these comorbidities are still unclear, which has hindered diagnosis and monitoring. We have investigated the effect of TBI in a PR5 tauopathy model using Diffusion Tensor Imaging and histology. We concluded that neuroinflammation is a key trigger in worsening taupathy accumulation and MRI can detect this cascade of events at an early stage. This study will enhance our understanding, not only of the effect of TBI progression in AD, but the potential of MRI for translational purposes.
Introduction:
Traumatic Brain Injury (TBI) and Alzheimer’s Disease (AD), have been shown to have common accumulation of neurofibrillary tau tangles and amyloid-beta plaques (Aβ), the classical pathological hallmarks of AD, suggestive of a mechanistic link between TBI and AD1-3. There is emerging evidence that a key interplay between microglia activation, Aβ accumulation and tau aggregation that is exacerbated following a TBI where neuroinflammation is a key factor in triggering the progression. Diffusion Tensor Imaging (DTI) has surfaced as a noninvasive modality very sensitive to microstructural changes induced in the tissue and changes in DTI metrics have been shown to mirror underlying inflammatory responses in the brain. While measures of diffusion have been extensively studied in AD, changes in brains prone to developing AD following a TBI are yet to be investigated. Here, we hypothesized that neuroinflammation is a key trigger for faster progression of AD pathology following a TBI. This study shows that DTI can mirror the cascade of events triggered by TBI in a tau model of AD.Method
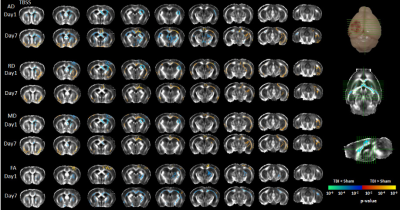
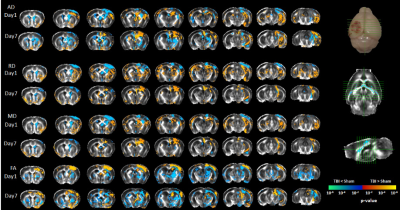
Design: PR5 transgenic mice (n=36, age 8–9 months old, 19 male; 21–35 g) were used. Animals were randomly divided into 4 groups: 2 TBI, 2 sham groups (n=9 per group), animals were scanned and sacrificed at 1 and 7 days post-injury. A single moderate cortical injury was induced in the left hemisphere with a controlled cortical impactor in TBI animals; sham groups underwent only craniotomy. MRI: T2-weighted and DTI were acquired using 9.4 Biospec equipped with a cryo probe (Bruker, Germany). Animals were anesthetized before scanning. A T2-TurboRARE sequence was acquired (RareFactor=8, TR= 7200ms; TE= 39ms; Averages=4; FOV=19.2x19.2x15.2mm, slice thickness=0.3mm) and axial echo-planar imaging DTI scan (TR= 10000ms; TE=25ms; 48, 0.3mm slices; FOV=18x18x15.6 mm; 33 noncollinear directions, (b=750;2000 s/mm2); 4 b0) and reverse phase with 3 directions. DTI was pre-processed and analysed using FSL (5.0.9)4. DTI maps were registered to study specific maps generated from initial normalization to Australian Mouse Brain Mapping Consortium (AMBMC) template. TBI and sham were compared at each time-point with unpaired t-test using non-parametric estimation (FDR corrected p<0.05, cluster size > 50 voxels). Tract-based spatial statistics (TBSS) was performed for white-matter changes. Histology: Immunofluorescence was performed on free-floating serial sections for astrocytes (glial fibrillary acidic protein (GFAP)), microglia (Iba-1), total tau and phospho-tau (MC1). Images were acquired using V-Slide scanner (MetaSystems) by Zeiss Axio Imager Z2. The total area with pathology per-section/per-animal was calculated using Imaris software (Imaris; Bitplane, Zurich, Switzerland). Groups were compared with unpaired nonparametric student t-test.Results
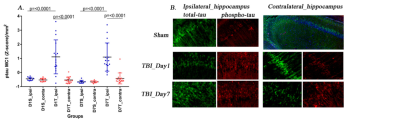
A significant increase in micro- and astro-glial cell reactivity post-injury in the TBI group vs sham. Iba-1 immunoreactivity was significantly high after 24 hrs and increased further up to 7 days post-injury (Fig. 1). A significant increase in (GFAP) immunoreactivity for astrocytes was evident after 1 week (Fig. 1). Increase in total and phospho-tau levels were observed in TBI animals (Fig. 2). Changes in diffusivity measures and anisotropy were observed in regions with high immune cells. Decreased axial-diffusivity (AxD) and Fractional Anistropy (FA) in white-matter (Fig. 3) and thalamus (Fig. 4) after 24 hr and remained low up to day 7. Radial diffusivity (RD) and mean diffusivity (MD) were increased after 24 hr and persisted till day 7.Discussion
Neuroinflammation is common to both TBI and AD5. Reduced AxD and FA, and increased RD, MD at 1 day post injury is characterized by microglial activation in the white-matter. However, RD and MD changes were acute in high immune response regions, possibly due to abridged intra-axonal water diffusivity following impact or the higher restriction due to the presence of infiltrating cells. This reflects DTI being a marker for microglial activation which furthermore, could be a marker of tau given we observed increased tau pathology after 24 hr, which is consistent with the previous findings6,7.
From this study, we can conclude that microglia are key to the disease pathogenesis, while astrocyte reactivity starts at a later stage that worsens the tau pathology. FA, AD and RD mirrors the underlying inflammatory changes triggered and is demonstrated to be a promising modality in reflecting brain progression following TBI.
Acknowledgements
This work was supported by Motor Accident Insurance Commission (MAIC) (Grant: 2014000857), The Queensland Government, Australia for the research grant to FN. NS acknowledges UQI for the Ph.D. scholarship. We thank the Australian Government support through NCRIS and the National Imaging Facility for the operation of 9.4T MRI at CAI.References
1. Faden, A.I. and Loane, D.J. (2015). Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 12, 143-1500.
2. Gerson, J., Castillo-Carranza, D.L., Sengupta, U., Bodani, R., Prough, D.S., DeWitt, D.S., Hawkins, B.E. and Kayed, R. (2016). Tau Oligomers Derived from Traumatic Brain Injury Cause Cognitive Impairment and Accelerate Onset of Pathology in Htau Mice. J Neurotrauma 33, 2034-2043.
3. Washington, P.M., Morffy, N., Parsadanian, M., Zapple, D.N. and Burns, M.P. (2014). Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. J Neurotrauma 31, 125-134.
4. Jenkinson, M., Beckmann, C.F., Behrens, T.E., Woolrich, M.W. and Smith, S.M. (2012). Fsl. Neuroimage 62, 782-790.
5. Fleminger, S., Oliver, D.L., Lovestone, S., Rabe-Hesketh, S. and Giora, A. (2003). Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74, 857-862.
6. Bolos, M., Llorens-Martin, M., Perea, J.R., Jurado-Arjona, J., Rabano, A., Hernandez, F. and Avila, J. (2017). Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol Neurodegener 12, 59.
7. Leyns, C.E.G. and Holtzman, D.M. (2017). Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener 12, 50.
Figures