0082
Advanced diffusion-weighted imaging reveals distinct neuropathological processes in concussed youth1Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 2Computer Science, University of Sherbrooke, Sherbrooke, QC, Canada, 3Pediatrics, Montreal Children's Hospital, Montreal, QC, Canada
Synopsis
Because concussions are undetectable by conventional medical imaging, their diagnosis is dependent on symptoms, which can be unreliable. Conventional diffusion-weighted imaging (DWI) can detect abnormalities in concussed individuals, but lack specificity and hence cannot provide information about the underlying neuropathology, especially during the sub-acute stage of concussions where different neuropathologies occur simultaneously. In this study, we used emerging DWI methods to disambiguate the neuropathological basis of a common concussive symptom, memory problems. We found evidence of different white-matter neuropathologies in concussed youth which contributed differently to memory problems. This study is an important step towards developing neuropathologically-informed biomarkers of concussion.
Introduction
Concussions are challenging to clinicians and researchers because of their massive prevalence and wide-ranging sequelae. Because concussions are not detectable via conventional medical imaging (Lewine, Davis, Sloan, Kodituwakku, & Orrison, 1999; Shenton et al., 2012; Sundman, Doraiswamy, & Morey, 2015; Yuh et al., 2013), their diagnosis relies heavily on patient-reported symptoms, which can often be unreliable due to underreporting (Meier et al., 2015) or to brain abnormalities that persist after symptom resolution (Churchill et al., 2017). Shearing forces transmitted across the brain during impact often result in damage localized to the brain’s white matter (Hayes, Bigler, & Verfaellie, 2016). Previous diffusion-tensor imaging (DTI) studies have found differences between concussed individuals and healthy controls (Hulkower et al., 2013), suggesting that diffusion-weighted imaging (DWI) is a promising technique to diagnose concussions. However, DTI faces certain methodological limitations that prevent it from fully capturing the neuropathological diversity of concussions. The neuropathological impact of concussions in white matter has been investigated in animal studies and includes inflammation, demyelination, and loss of axons (Bigler & Maxwell, 2012). At the sub-acute stage, these neuropathological processes can overlap, yielding often-contradictory results that cannot be distinguished using DTI (Dodd, Epstein, Ling, & Mayer, 2014). The present study sought to use emerging DWI methods to clarify the neuropathological impact of concussions at the sub-acute stage. Because of the wide scope of this question, we restricted the focus of the study to one particular common symptom of concussions: working memory problems. Hence, the present study sought to improve understanding of the neuropathology involved in working-memory problems associated with concussions.Methods
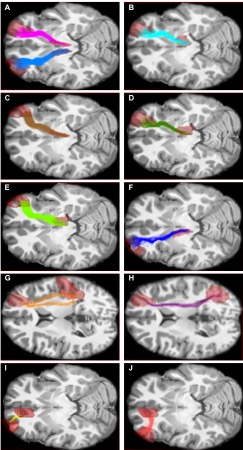
We studied a sample of 62 children and adolescents, which consisted of 46 healthy controls and 16 concussed youth, all scanned between 9 and 90 days after their injury (during the sub-acute stage). Diffusion weighting was performed along 99 non-collinear directions using a b-value of 1000 s/mm2. Ten b=0 s/mm2 images were acquired as reference. We used constrained-spherical deconvolution (CSD) to model fiber orientation distribution functions (fODFs) (Descoteaux, Deriche, Knosche, & Anwander, 2009; Tournier, Calamante, & Connelly, 2007; Tournier, Calamante, Gadian, & Connelly, 2004), as well as particle-filtering tractography with anatomical priors (Girard, Whittingstall, Deriche, & Descoteaux, 2014) to isolate 11 working memory tracks (Figure 1). Along these tracks, we measured Fractional Anisotropy (FA), Axial Diffusivity (AD), Mean Diffusivity (MD), Radial Diffusivity (RD), track volume, and Apparent Fiber Density (AFD). We also used AMICO (Daducci et al., 2015) to compute Free Water fraction (FW) along these tracks.Results
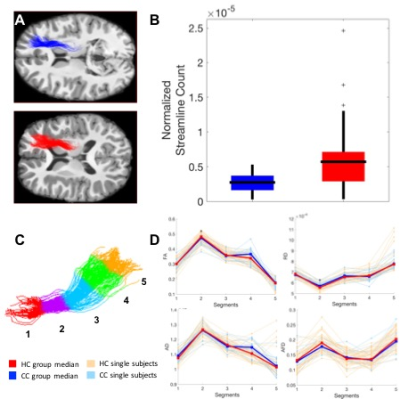
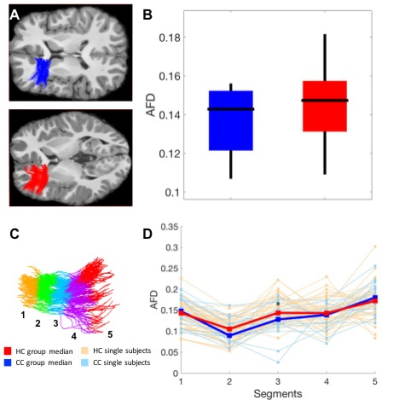
Compared to healthy controls, in two tracks connecting the right thalamus to the right dorsolateral-prefrontal cortex (DLPFC), concussed youth had decreases in streamline count across the entire track, as well as decreases in FA, increases in MD, RD, and FW in one segment of each track (Figure 2). In another track connecting the left anterior-cingulate cortex with the left DLPFC, concussed youth had decreases in FA and AFD across the entire track, as well decreases in AFD in one segment, and increases in FW in another (Figure 3). No significant differences in the Number of Fiber Orientations (NuFO) were found in these tracks. Significant microstructural measures were entered into linear regressions predicting working memory accuracy. FA and MD in the same segment of one thalamo-prefrontal track significantly predicted working memory accuracy, and so did RD and FW in different segments of the other thalamo-prefrontal track. In the cingulo-prefrontal track, AFD did not predict working memory accuracy. FW values in all three tracks were highly correlated. Structural differences and tractography reconstructions were validated using test-retest analyses.Discussion
The microstructural differences in both thalamo-prefrontal tracks were suggestive of myelin loss. A lack of NuFO differences in these tracks suggests that changes in underlying fiber configurations are unlikely to play a role in these results, further supporting the possibility that these differences are due to myelin changes. In the cingulo-prefrontal track, microstructural changes were instead suggestive of axonal loss. The highly correlated FW increases in all three tracks were suggestive of generalized edema. White-matter neuropathology in the two thalamo-prefrontal tracks, but not the cingulo-prefrontal track, appeared to play a key role in working memory deficits in concussion.Conclusion
Leveraging advances in diffusion modelling, tractography, and tractometry, this study is the first to capture, with a single imaging modality, the neuropathological heterogeneity of pediatric concussions in vivo. These results provide a better understanding of the neuropathology of working memory in concussions, which appears to involve alterations in myelin structure in thalamo-prefrontal pathways. This study is an important step towards developing neuropathologically-informed biomarkers of concussion in children.Acknowledgements
No acknowledgement found.References
Bigler, E. D., & Maxwell, W. L. (2012). Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav, 6(2), 108-136. doi:10.1007/s11682-011-9145-0
Churchill, N. W., Hutchison, M. G., Richards, D., Leung, G., Graham, S. J., & Schweizer, T. A. (2017). Neuroimaging of sport concussion: persistent alterations in brain structure and function at medical clearance. Scientific Reports, 7(1), 8297. doi:10.1038/s41598-017-07742-3
Daducci, A., Canales-Rodriguez, E. J., Zhang, H., Dyrby, T. B., Alexander, D. C., & Thiran, J. P. (2015). Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage, 105, 32-44. doi:10.1016/j.neuroimage.2014.10.026
Descoteaux, M., Deriche, R., Knosche, T. R., & Anwander, A. (2009). Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Transactions on Medical Imaging, 28(2), 269-286. doi:10.1109/TMI.2008.2004424
Dodd, A. B., Epstein, K., Ling, J. M., & Mayer, A. R. (2014). Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. Journal of Neurotrauma, 31(14), 1235-1248. doi:10.1089/neu.2014.3337
Girard, G., Whittingstall, K., Deriche, R., & Descoteaux, M. (2014). Towards quantitative connectivity analysis: reducing tractography biases. Neuroimage, 98, 266-278. doi:10.1016/j.neuroimage.2014.04.074
Hayes, J. P., Bigler, E. D., & Verfaellie, M. (2016). Traumatic Brain Injury as a Disorder of Brain Connectivity. Journal of the International Neuropsychological Society, 22(2), 120-137. doi:10.1017/S1355617715000740
Hulkower, M. B., Poliak, D. B., Rosenbaum, S. B., Zimmerman, M. E., & Lipton, M. L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR: American Journal of Neuroradiology, 34(11), 2064-2074. doi:10.3174/ajnr.A3395
Lewine, J. D., Davis, J. T., Sloan, J. H., Kodituwakku, P. W., & Orrison, W. W., Jr. (1999). Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR: American Journal of Neuroradiology, 20(5), 857-866.
Meier, T. B., Brummel, B. J., Singh, R., Nerio, C. J., Polanski, D. W., & Bellgowan, P. S. (2015). The underreporting of self-reported symptoms following sports-related concussion. Journal of Science and Medicine in Sport, 18(5), 507-511. doi:10.1016/j.jsams.2014.07.008
Shenton, M. E., Hamoda, H. M., Schneiderman, J. S., Bouix, S., Pasternak, O., Rathi, Y., . . . Zafonte, R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav, 6(2), 137-192. doi:10.1007/s11682-012-9156-5
Sundman, M., Doraiswamy, P. M., & Morey, R. A. (2015). Neuroimaging assessment of early and late neurobiological sequelae of traumatic brain injury: implications for CTE. Frontiers in Neuroscience, 9, 334. doi:10.3389/fnins.2015.00334
Tournier, J. D., Calamante, F., & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage, 35(4), 1459-1472. doi:10.1016/j.neuroimage.2007.02.016
Tournier, J. D., Calamante, F., Gadian, D. G., & Connelly, A. (2004). Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage, 23(3), 1176-1185. doi:10.1016/j.neuroimage.2004.07.037
Yuh, E. L., Mukherjee, P., Lingsma, H. F., Yue, J. K., Ferguson, A. R., Gordon, W. A., . . . Investigators, T.-T. (2013). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Annals of Neurology, 73(2), 224-235. doi:10.1002/ana.23783
Figures