Placenta & Pregnant Patient
1Assistance Publique-Hôpitaux de Paris, Hôpital Louis Mourier, France, 2INSERM, U970, Sorbonne Paris Cite, Paris Cardiovascular Research Center – PARCC, Paris, France, 3EA FETUS, 7328 & LUMIERE PLATFORM, Université Paris-Descartes, Paris, France, 4Assistance Publique-Hôpitaux de Paris, Hôpital Robert Debré, France, 5Assistance Publique-Hôpitaux de Paris, Hôpital Necker-Enfants Malades, France
Synopsis
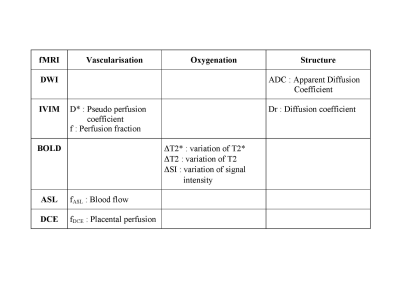
Understanding placental functions remains largely concealed from non-invasive in vivo investigations, although placental insufficiency is responsible for most failures in pregnancy. Functional MRI offers new perspectives for placental imaging. The vascularisation can be studied with IVIM and the pseudo perfusion coefficient (D*) and the perfusion fraction (f), and with ASL and the blood flow (fASL). DCE MRI also provides a definite assessment of perfusion but requires an exogenous contrast agent. The oxygenation can be studied with BOLD. The placental structure can be studied with DWI and the apparent diffusion coefficient (ADC), and with IVIM and the diffusion coefficient (Dr).
Purpose:
The placenta is the key organ for the fetal development. It is a regulatory organ which has involvement far beyond nutritive and respiratory functions, to also encompass endocrine and immune system regulation. Understanding placental functions remains largely concealed from non-invasive in vivo investigations, although placental insufficiency is responsible for most failures in pregnancy. Magnetic Resonance Imaging (MRI) has been performed in pregnancy for over 30 years, and is mainly a second-line imaging modality after Ultrasonography. Recent developments in MRI offer new perspectives for placental imaging, using functional MRI (fMRI) tools to explore vascularisation, oxygenation and metabolism.
Methods :
FMRI of the placenta adds functional information to anatomical data in vivo and non-invasively. A large panel of the fMRI techniques can be used to further explore placental functions related to vascularisation, oxygenation, metabolism, through various enhancement processes. Available methods will be described. An overview of the theory, practice, and potential applications of each fMRI will be exposed and discussed.
Results :
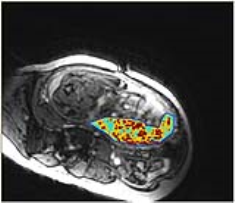
Diffusion weighting Imaging (DWI) and Intra Voxel Incoherent Motion (IVIM) MRI study the movement of water molecules within tissues and provide information about vascularization and tissular structure. The Apparent Diffusion Coefficient (ADC) is obtained with DWI and is related to tissular structure. Three quantitative parameters are available with IVIM. The pseudo perfusion coefficient (D*) and the perfusion fraction (f) are related to vascularization. The diffusion coefficient (Dr) is related to tissular structure. The Apparent Diffusion Coefficient (ADC) and the perfusion fraction (f) are significantly decreased in growth restriction placentas when compared to normal pregnancies and could be used to detect intra uterine growth retardation (IUGR). Blood Oxygen Level Dependent (BOLD) MRI uses the hemoglobin (Hb) as an endogenous contrast agent. The magnetic properties of Hb depend on its oxygen saturation, and modifications of T2* (ΔT2*), T2 (ΔT2), signal intensity (ΔSI) can be depicted when the oxygenation status of the organ of interest is modified. The variation of T2*, T2 or signal intensity can be used to assess placental oxygenation and response to oxygenation. The placenta has already been studied in physiological conditions during maternal hyperoxia, demonstrating differences between the chorionic and the basal plates of the placenta. A clinical research trial designed to evaluate the use of BOLD MRI in pregnant women as a non-invasive diagnostic tool to detect placental insufficiency and differentiate healthy fetuses from those with IUGR is underway (figure). Arterial Spin Labeling (ASL) MRI relies on magnetically labeled water to quantify the placental blood flow (fASL). This technique was the first fMRI technique used to study the human placenta in 1998 : fASL in normal pregnancy is 176 ± 91 ml.min-1.100 g-1 and decreases in cases with IUGR. Dynamic Contrast Enhanced MRI (DCE-MRI) is best able to quantify placental perfusion (fDCE), permeability and blood volume fractions. The enhancement of the placenta after intravenous injection of a Gadolinium-based contrast agent is compared to the enhancement of the arterial input function using compartimental model. However, the use of Gadolinium-based contrast agents during pregnancy is still limited to situations when the benefits outweigh the potential risks.
Discussion :
FMRI can be used to explore the vascularisation, the oxygenation and the structure of the placenta. The vascularisation can be studied with IVIM and the pseudo perfusion coefficient (D*) and the perfusion fraction (f), and with ASL and the blood flow (fASL). DCE MRI also provides a definite assessment of perfusion but requires the use of an exogenous contrast agent. The oxygenation can be studied with BOLD and the measurement of SI or T2* during two different oxygenation status. The placental structure can be studied with DWI and the apparent diffusion coefficient (ADC), and with IVIM and the diffusion coefficient (Dr). FMRI will be used to investigate normal pregnancy, IUGR, and pre ecclampsia. In normal conditions, basic values of ADC, the perfusion fraction (f) and the placental blood flow (fASL) are available. In IUGR, ADC, perfusion fraction f and T2 decrease. The perfusion fraction (f) can be useful in pre ecclampsia.
Conclusion :
Modern imaging of the placenta will integrate fMRI techniques in the follow up of pregnancy as numerous biomarkers will become available. The objectives of such modern imaging will be to improve the detection of placental insufficiency and the management of high-risk fetuses. However, the respective contribution of each technique remains to be defined. A plateform dedicated to human foeto-placental research (LUMIERE) will be created soon and will allow further research in this area.
Acknowledgements
No acknowledgement found.References
Functional imaging of the human placenta with magnetic resonance. Siauve N, Chalouhi GE, Deloison B, Alison M, Clement O, Ville Y, Salomon LJ. Am J Obstet Gynecol. 2015 Oct;213 :S103-14.
Use of intravoxel incoherent motion MR imaging to assess placental perfusion in a murine model of placental insufficiency. Alison M, Chalouhi GE, Autret G, Balvay D, Thiam R, Salomon LJ, et al. Invest Radiol. 2013 Jan;48(1):17–23.
Assessment of human placental perfusion by intravoxel incoherent motion MR imaging. Siauve N, Hayot PH, Deloison B, Chalouhi GE, Alison M, Balvay D, Bussières L, Clément O, Salomon LJ.. J Matern Fetal Neonatal Med. 2017 Oct 3:1-8.
Diffusion-weighted MR imaging of the placenta in fetuses with placental insufficiency. Bonel HM, Stolz B, Diedrichsen L, Frei K, Saar B, Tutschek B, et al. Radiology. 2010;257(3):810–9.
In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Moore RJ, Strachan BK, Tyler DJ, Duncan KR, Baker PN, Worthington BS, et al. Placenta. 2000;21(7):726–32.
In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging. Aimot-Macron S, Salomon LJ, Deloison B, Thiam R, Cuenod CA, Clement O, et al. Eur Radiol. 2013 May;23:1335–42.
Fetoplacental oxygenation in an intrauterine growth restriction rat model by using blood oxygen level-dependent MR imaging at 4.7 T. Chalouhi GE, Alison M, Deloison B, Thiam R, Autret G, Balvay D, et al. Radiology. 2013 Oct;269:122–9.
Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI. Sørensen A, Peters D, Simonsen C, Pedersen M, Stausbøl-Grøn B, Christiansen OB, et al. Prenat Diagn. 2013 Feb;33(2):141–5.
The measurement of fetal liver T(*)(2) in utero before and after maternal oxygen breathing: progress towards a non-invasive measurement of fetal oxygenation and placental function. Semple SI, Wallis F, Haggarty P, Abramovich D, Ross JA, Redpath TW, et al. Magn Reson Imaging. 2001 Sep;19(7):921–8.
In vivo perfusion measurements in the human placenta using echo planar imaging at 0.5 T. Gowland PA, Francis ST, Duncan KR, Freeman AJ, Issa B, Moore RJ, et al. Magn Reson Med. 1998;40(3):467–73.
Placental MRI. Gowland P. Semin Fetal Neonatal Med. 2005 Oct;10(5):485–90.
Non-invasive mapping of placental perfusion. Francis ST, Duncan KR, Moore RJ, Baker PN, Johnson IR, Gowland PA. Lancet. 1998;351(9113):1397–9.
Placental perfusion MR imaging with contrast agents in a mouse model. Salomon LJ, Siauve N, Balvay D, Cuenod CA, Vayssettes C, Luciani A, et al. Radiology. 2005 Apr;235(1):73–80.