Diffusion-weighted MR Spectroscopy
1Center of neuroimaging research (CENIR) - ICM, Paris, France
Synopsis
Diffusion-weighted 1H MR spectroscopy (DW-MRS) provides unique cell-specific and compartment-specific microstructural information based on the diffusion properties of intracellular metabolites in brain tissue. In this talk, the basic aspects of DW-MRS data acquisition and processing will be presented, together with some clinical and preclinical applications.
Learning Objectives
· Optimised incorporation of diffusion weighting in standard single-voxel MRS sequences
· Major sources of error in DW-MRS data and their correction
· Obtaining standard diffusion metrics from DW-MRS data
Introduction
Diffusion-weighted MR spectroscopy (DW-MRS) enables the investigation of the intra-cellular environment through the measurement of the diffusion properties of several brain metabolites in vivo [1-3]. In contrast to water molecules, which are present in all tissue compartments (intra and extra-cellular), metabolites are unique probes of the intra-cellular space, and can thus provide specific information on tissue properties, such as cytosol viscosity, molecular crowding, tortuosity of the intra-cellular compartments, cell size and geometry. Moreover, thanks to the preferential location of certain metabolites in specific cell types, DW-MRS can help disentangling the effect of multiple pathological processes affecting tissue microstructure, and complement more sensitive but less specific methods such as diffusion tensor imaging (DTI). Although very promising, DW-MRS also poses several challenges, which may limit the incorporation of this technique in standard MR protocols: DW-MRS data acquisition is severely affected by eddy currents generated by the strong diffusion weighting gradients, and the signal is highly susceptible to physiological fluctuations, cardiac pulsation, and motion. As a result, the robustness and reproducibility of DW-MRS strongly depend on a highly optimised pipeline of acquisition and processing.DW-MRS pulse sequences
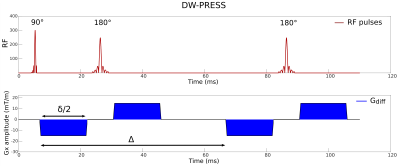
DW-MRS sequences can be derived from standard MRS sequences via the addition of magnetic field gradients pulses to sensitize the NMR signal to diffusion. At the moment, the most commonly used techniques for spatial localization are point-resolved spectroscopy (PRESS) [4], stimulated echo acquisition mode (STEAM) [5], and localization by adiabatic selective refocusing (LASER) [6] and their variants. The proper sequence to employ in a DW-MRS experiment has to be chosen depending on several factors, including an optimal compromise between signal-to-noise ratio (SNR), precision of metabolite quantification, and the required conditions on the DW parameters for the modeling of the diffusion data. While spin-echo sequences provide higher SNR for a given echo time (TE), DW-STEAM allows for long diffusion times (Δ) keeping short the TE, and it has been shown to better quantify diffusion of J-coupled metabolites [7]. If the short gradient pulse duration (δ) condition (δ << Δ) is not strictly required, and there is no need for long diffusion times, a full bipolar PRESS sequence (Fig. 1) offers a diffusion-weighting scheme with several advantages, such as minimization of eddy currents and minimization of the interaction between diffusion gradients and ‘background gradients’ reflecting the inhomogeneity of B0. In addition, the bipolar scheme allows to maximize the b value achievable for a given TE, which is an important factor especially for applications on clinical scanners, where the maximum available gradient strength is often limited. The use of fully adiabatic LASER [6] or partially adiabatic sLASER [8] coupled with diffusion gradients is advantageous when the B1 field is highly inhomogeneous.Data processing
In DW-MRS, several spectra are acquired for each DW
condition and then averaged. Phase variations caused by bulk motion during the
experiment induce a signal loss after averaging which is exacerbated at high b
values, thus yielding an overestimation of the metabolite apparent diffusion
coefficient (ADC) values. It is therefore crucial to individually phase-correct
spectra before averaging. Amplitude fluctuations at high b values caused by non-translational
motion or by compressive motion due to cardiac and cerebrospinal fluid
pulsation can also result in signal loss and consequent overestimation of
metabolite ADCs. These fluctuations can be minimized by synchronizing the
acquisition to the cardiac cycle using a pulse peripheral unit (PPU) or an
electrocardiograph device (ECG). Retrospective removal of spectra with
intensity below a certain threshold can be also used to overcome the
detrimental effect induced by amplitude fluctuations, which can be hardly
controlled for example in experiments employing ultra-long diffusion times. In
DW-MRS, the effect of eddy currents on the line shape of the spectra is
amplified by the presence of strong diffusion gradients. A separate acquisition
without water suppression performed in the same region of interest and under
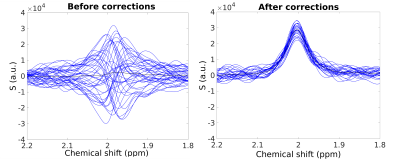
the same DW conditions is necessary for proper eddy current correction. Fig.
2 shows an example of DW spectra acquired at 3 T for b = 3300 s/mm2, plotted before and
after phase, frequency, and eddy current corrections.
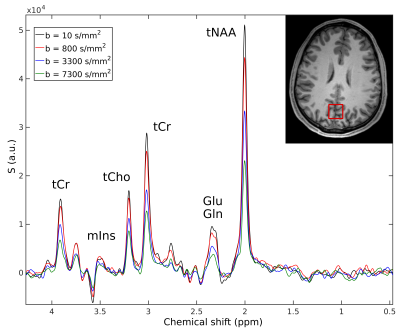
The
signal drop in DW spectral peaks (Fig. 3) related to the diffusion of specific
metabolites can be quantified in order to derive diffusion-weighted signal
attenuation curves as a function of the b value.
Metabolite diffusion properties can be derived by analyzing
the corresponding signal decay with either simple mono or bi-exponential models,
or using more sophisticated biophysical models when the acquisition scheme
allows for it (see for example [9-12]). Brain cell morphology can be
investigated in vivo by measuring metabolite ADC time dependence at ultra-long
diffusion times [13-14].Clinical applications
The specificity of DW-MRS measures enables to distinguish pathological processes occurring simultaneously in diseased brain. So far, DW-MRS has been applied to study normal aging, cerebral ischemia, neurodegenerative, and psychiatric disorders (see refs 2-3 for exhaustive review). Notably, the diffusion of the neuronal marker N-acetylaspartate (NAA) has been suggested to reflect the pure intra-axonal damage in white matter diseases [15], while the diffusion of choline compounds (tCho) may be used as marker of inflammation and glial cells alterations [16].Acknowledgements
No acknowledgement found.References
[1] Nicolay K et al, Diffusion NMR spectroscopy. NMR Biomed, 14:94-111 (2001).
[2] Ronen I and Valette J, Diffusion-weighted magnetic resonance spectroscopy. eMagRes. Volume 4. John Wiley & Sons, Ltd, Chichester, United Kingdom, p 733-750 (2015).
[3] Palombo M, Shemesh N, Ronen I, Valette J, Insights into brain microstructure from in vivo DW-MRS, NeuroImage (2017), doi: 10.1016/j.neuroimage.2017.11.028.
[4] Bottomley PA, Spatial localization in NMR spectroscopy in vivo, Ann. N. Y. Acad. Sci., 508:333-48 (1987).
[5] Frahm J et al, Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites, MRM, 11: 47-63 (1989).
[6] Garwood M and DelaBarre L, The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson, 153:155-77 (2001).
[7] Deelchand DK et al, Apparent diffusion coefficients of the five major metabolites measured in the human brain in vivo at 3T, MRM, 79:2896-2901 (2018).
[8] Scheenen TW at al, Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. MRM, 59:1 (2008).
[9] Palombo M et al, Modeling diffusion of intracellular metabolites in the mouse brain up to very high diffusion-weighting: diffusion in long fibers (almost) accounts for non-monoexponential attenuation, MRM, 77:343-350 (2017).
[10] Palombo M. et al, Can we detect the effect of spines and leaflets on the diffusion of brain intracellular metabolites?. NeuroImage (2017). In press doi.org/10.1016/j.neuroimage.2017.05.003
[11] Shemesh et al, Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nature communications, 5:4958 (2014).
[12] Ronen I et al, Microstructural organization of axons in the human corpus callosum
quantified by diffusion-weighted magnetic resonance spectroscopy of
N-acetylaspartate and post-mortem histology. Brain Struct Funct, 219(5):1773-85 (2014).
[13] Palombo M et al, New paradigm to assess brain cell morphology by diffusion-weighted MR spectroscopy in vivo, PNAS, 113:6671-6676 (2016).
[14] Valette J et al, Brain metabolite diffusion from ultra-short to ultra-long time scales: what do we learn, where should we go? Frontiers in neuroscience, 12:2, (2018).
[15] Wood ET et al, Investigating Axonal Damage in Multiple Sclerosis by Diffusion Tensor Spectroscopy, J Neurosci, 32:6665-6669 (2012).
[16] Ercan E et al, Glial and axonal changes in systemic lupus erythematosus measured with diffusion of intracellular metabolites, Brain, 139:1447-57 (2016).
Figures