Functional MR Spectroscopy
1Bangor University, United Kingdom
Synopsis
Proton MRS is often thought of as a static measure. In the past 10 years however, this view has been challenged by several studies showing that it is actually sensitive to fluctuations in neurometabiolites as a result of neural activity. This technique of "functional" MRS (fMRS) is not new, having been around since 1991, however with the ready availability of 3T or higher MRI systems, improved acquisition techniques and accurate fitting packages, fMRS has seen renewed interest. A discussion of the main technique, the result that might be expected and experimental considerations for fMRS will be presented using Glutamate as the model neurometabolite. Attendees should come away with a renewed appreciation for the role 1H-fMRS may play in understanding neural activity and function.
Target audience
Aimed at research scientists and clinicians who have an interest in applying proton MRS in the study of brain function. Useful for junior as well as Senior researchers.Objectives
Provide attendees with an understanding of: the current state of the field “Functional” Magnetic Resonance Spectroscopy (fMRS) using proton (1H) MRS; some of the current controversies; issues researchers should consider when designing 1H-fMRS studies; and interpretation of the same.Purpose
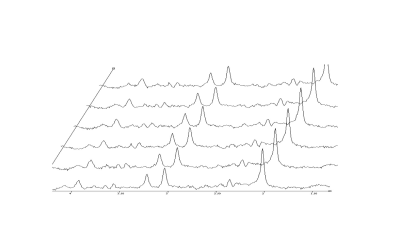
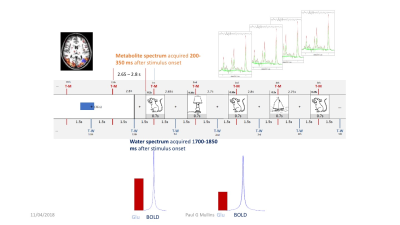
Proton magnetic resonance spectroscopy is a powerful tool to investigate neurochemistry and physiology in vivo. Recently researchers have started to use MRS to measure neurotransmitter changes related to neural activity, so called functional MRS (fMRS). Particular interest has been placed on measuring glutamate changes associated with neural function, but other neuro metabolites are also of interest. This session will discuss 1H-fMRS, and includes meta-analyses of the relative size of glutamate changes seen in fMRS, and the impact experimental design and stimulus paradigm may have on these changes as an example of work in the field.Methodology
A brief over view of fMRS will be provided. Then to illustrate the types of effects seen, results of a meta-analysis of current glutamate based fMRS studies will be presented.Results and Discussion
On average glutamate has been reported to increase by 6.97 % (±1.739%) in response to neural activation in fMRS studies. However, factors of experimental design may have a large impact on the size of these changes. For example, an increase of 4.749% (±1.45 %) is seen in block studies compared to an increase of 13.429% (± 3.59) in studies using event related paradigms. The stimulus being investigated also seems to play a role with prolonged visual stimuli showing a small mean increase in glutamate of 2.318 % (± 1.227%) while at the other extreme, pain stimuli show a mean stimulation effect of 14.458 % (± 3.736%).
These differences are discussed with regards to possible physiologic interpretations, as well experimental design implications. Considerations of what the results of previous studies may have on experimental design will be proposed, and a brief discussion of the role of neural adaptation, BOLD effects and measurement parameters will be included.The ability of fMRS to be combined with other measures of neural activity will be discussed along with the other neurometabiolites that are currently being investigated.
Conclusion
MRS is more than just a measure of static neurochemistry. Investigation of neurotransmitter and neurometabolite dynamics is possible – opening up even more windows on the basic functioning of the brain. More work is needed in the realm of interpretation of the results currently being reported, and optimising experimental design - including acquisition parameters, analysis and fitting, and how best to combine data. It is hoped that future researchers will take this technique and apply it to better elucidate the inner workings of the brain and cognition at even finer detail not only in the healthy human brain, but also in conditions where neurotransmitter dysfunction is thought to play a major role.Acknowledgements
No acknowledgement found.References
Reviews:
Mullins, P. G. (2017). Towards a theory of functional magnetic resonance spectroscopy (fMRS): A meta-analysis and discussion of using MRS to measure changes in neurotransmitters in real time. Scandinavian Journal of Psychology, 1–13. http://doi.org/10.1111/sjop.12411
Jelen, L. A., King, S., Mullins, P. G., & Stone, J. M. (2018). Beyond static measures: A review of functional magnetic resonance spectroscopy and its potential to investigate dynamic glutamatergic abnormalities in schizophrenia. Journal of Psychopharmacology, 1, 026988111774757–12. http://doi.org/10.1177/0269881117747579
Selected relevant papers:
Apšvalka, D., Gadie, A., Clemence, M., & Mullins, P. G. (2015). Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. NeuroImage,118, 292–300. http://doi.org/10.1016/j.neuroimage.2015.06.015
Bednařík, P., Tkáč, I., Giove, F., DiNuzzo, M., Deelchand, D. K., Emir, U. E., et al. (2015). Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. Journal of Cerebral Blood Flow Metabolism, 35(4), 601–610. http://doi.org/10.1038/jcbfm.2014.233
Bednařík, P., Tkáč, I., Giove, F., Eberly, L. E., Deelchand, D. K., Barreto, F. R., & Mangia, S. (2017). Neurochemical responses to chromatic and achromatic stimuli in the human visual cortex. Journal of Cerebral Blood Flow Metabolism, 271678X17695291. http://doi.org/10.1177/0271678X17695291
Chen, C., Sigurdsson, H. P., Pépés, S. E., Auer, D. P., Morris, P. G., Morgan, P. S., et al. (2017). Activation induced changes in GABA: functional MRS at 7 T with MEGA-sLASER. NeuroImage, 156, 207–213. http://doi.org/10.1016/j.neuroimage.2017.05.044
Ip, I. B., Berrington, A., Hess, A. T., Parker, A. J., Emir, U. E., & Bridge, H. (2017). Combined fMRI-MRS acquires simultaneous glutamate and BOLD-fMRI signals in the human brain. NeuroImage, 155, 1–19. http://doi.org/10.1016/j.neuroimage.2017.04.030
Lally, N., Mullins, P. G., Roberts, M. V., Price, D., Gruber, T., & Haenschel, C. (2014). Glutamatergic correlates of gamma-band oscillatory activity during cognition: a concurrent ER-MRS and EEG study. NeuroImage, 85 Pt 2, 823–833. http://doi.org/10.1016/j.neuroimage.2013.07.049
Mangia, S., Giove, F., Tkáč, I., Logothetis, N. K., Henry, P.-G., Olman, C. A., et al. (2008). Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 29(3), 441–463. http://doi.org/10.1038/jcbfm.2008.134
Mangia, S., Tkáč, I., Gruetter, R., Van de Moortele, P.-F., Maraviglia, B., & Ugurbil, K. (2007). Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism,27(5), 1055–1063. http://doi.org/10.1038/sj.jcbfm.9600401
Mullins, P. G., Rowland, L. M., Jung, R. E., & Sibbitt, W. L. (2005). A novel technique to study the brain's response to pain: proton magnetic resonance spectroscopy. NeuroImage, 26(2), 642–646. http://doi.org/10.1016/j.neuroimage.2005.02.001