Looking from the Outside: Extracellular Diffusion
1NYU School of Medicine, United States
Synopsis
Diffusion in the extracellular space is considered as a function of an increasing diffusion time, equivalent to the coarse-graining of the cellular arrangement over an increasing diffusion length. The three major limits are covered: Short-time S/V limit; long-time limit of approaching the tortuosity asymptote; and the tortuosity limit. The relevant degrees of freedom of cell packing, distinct in each of the three limits, will be discussed.
Diffusion as coarse-graining
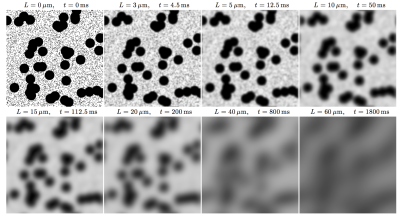
Diffusion in heterogeneous media is an example of coarse-graining [1], which is a procedure that averages the dynamics over finer-scale degrees of freedom to derive approximate effective dynamics at the coarser scales involving a minimal number of parameters. It can be thought of as a gradual “forgetting”, or homogenizing over the increasing diffusion length. To illustrate this concept, consider a two-dimensional model example of a two-scale structure, represented by randomly placed impermeable disks of two different radii, embedded into an NMR-visible space with diffusion coefficient D0, Fig. 1. For our qualitative purposes, this model system has all the necessary features of diffusion in the extra-axonal space transverse to a fiber tract, hindered by two types of axons; its three-dimensional analog would describe diffusion in the extra-cellular space in an isotropically packed tissue, such as a tumor.
We note from the outset, that a complete solution for the signal (the diffusion propagator) for all $$$q$$$ and $$$t$$$, as well as a solution for its cumulants, such as diffusion coefficient, kurtosis, etc, would involve knowing all positions and shapes of all cells in a sample; this is obviously impossible. The challenge of modeling is to identify the very few relevant degrees of freedom of the structural microgeometry of the tissue.
The following three regimes, (i)-(iii), can be then identified (see the following three sections), as function of the diffusion time, or of the corresponding diffusion length. In each of these regimes, the physics is conceptually different, and the relevant degrees of freedom which contribute the most to the observed $$$D(t)$$$ and the MRI signal, are distinct.
Regime (i): No coarse-graining has yet occurred
For times such that $$$L(t)S/V\ll1$$$, one observes the universal short-time limit [2] of the diffusion coefficient in $$$d$$$ spatial dimensions
$$D(t) \simeq D_0 \left( 1 - {4 \over 3\sqrt{\pi}\, d} {S\over V} \sqrt{D_0 t} \right)\qquad (1)$$
determined solely by the net surface-to-volume ratio $$$S/V$$$ of the cell walls. There is no dependence on the way the cells are arranged in space; in this sense, Eq. (1) provides a geometric measure of individual restrictions. Note that both intra- and extra-cellular water generally contributes to the overall $$$S/V$$$. In the red blood cell system, Eq. (1) was observed by Latour et al [3].
Practically, the short-time limit is most conveniently accessed with oscillating gradients; in the limit of large number of oscillations, the corresponding frequency-dependent diffusivity is given by [4]
$$D(\omega) \simeq D_0 \left( 1 - {1 \over d\sqrt{2}} {S\over V} \sqrt{D_0\over \omega}\right) . \qquad(2)$$
To determine cell sizes, one has to know the cell water fraction in addition to the estimated $$$S/V$$$; such relationship for the fiber phantom was used in ref [5] to validate Eq (2) on a clinical scanner.
However, even without the cell water fraction, the geometric parameter $$$S/V$$$ can be already informative. Reynaud et al [6] demonstrated the frequency scaling as in Eq. (2) in the mouse glioma model, and decoupled the free diffusivity $$$D_0$$$, that was shown to depend on the temperature of an ex-vivo tissue sample, from the geometric measure $$$S/V$$$ which was temperature-independent and correlated with cell density.
Regime (ii): Almost complete coarse-graining; approaching the tortuosity limit
In the limit of long diffusion times, corresponding to $$$L(t)\gg l_c$$$, where $$$l_c$$$ is the correlation length of the cell packing (practically, $$$l_c$$$ is of the order of cell size), the physics of $$$D(t)$$$ conceptually simplifies. Instead of supplying all microscopic degrees of freedom as in short-time subplots in Fig. 1, the relevant degrees of freedom are now contained to the slowly-varying diffusion coefficient $$$D(r)$$$ which is obtained by coarse-graining the medium over the scale $$$L(t)$$$, see the later panels of Fig. 1. In this case, the universal relation between the dynamical exponent $$$\vartheta$$$ in the instantaneous diffusion coefficient
$$ D_{\rm inst}(t) \simeq D_\infty + \mbox{const}\cdot t^{-\vartheta} \,, \quad \vartheta>0 \qquad(3)$$
and the structural exponent $$$p$$$ of the structure factor (the power-spectrum) of the restrictions $$$\Gamma(k) \sim k^p$$$, can be obtained [7]:
$$ \vartheta = (p+d)/2 .\qquad\qquad(4)$$
From this general theory, it follows in the practically relevant case of time-dependent diffusion transverse to axonal tracts ($$$d=2$$$), characterized by the short-range disorder in packing ($$$p=0$$$), that the $$$1/t$$$ tail in $$$D_{\rm inst}(t)$$$ corresponds to the logarithmic singularity [8] in the PGSE-measured cumulative
$$ D(t) \simeq D_\infty + A {\ln (t/t_c) \over t} \,, \quad t_c \sim {l_c^2\over D_\infty} . \qquad(5)$$
This nontrivial scaling was observed [9-11] in human white matter tracts. Parametrically, it overshadows the time-dependent contributions from the intra-axonal space. This means that at low $$$b$$$ used in the clinic, for which the extra-axonal signal is not sufficiently suppressed, we are practically mainly sensitive to the fiber packing correlation length of axons within a bundle, a measure of the extra-axonal space geometry $$$-$$$ instead of the inner axonal diameters.
In $$$d=3$$$ dimensions, the corresponding PGSE
$$D(t)\simeq D_\infty + {{\rm const} \over t} \qquad(6) $$
does not reveal the dynamical exponent $$$\vartheta$$$, while its OGSE analog would yield the corresponding $$$\sim \omega^{\vartheta}$$$ scaling, as long as $$$\vartheta \leq 2$$$ [7]. However, one can use the PGSE scaling (6) to extrapolate towards the tortuosity limit $$$D_\infty$$$, as it was done in the mouse glioma study [12], see also the recent review article [13] that comprehensively summarizes different regimes of the time-dependence and of the competition between the intra- and extra-axonal contributions for tumors.
Regime (iii): Complete coarse-graining, the tortuosity limit
This is the limit that is both the simplest and the most difficult. From the point of the functional form of the signal, the $$$t\to\infty$$$ limit is trivial: The signal from the extra-cellular compartment is Gaussian and possibly anisotropic, so it is fully characterized by its bulk (macroscopic) diffusion tensor. (The only case when this statement were to be incorrect would be the case of anomalous diffusion, which practically never arises in tissues [1].)
However, having just a single number (diffusivity in a given direction), or a tensor, is not satisfying: We would ideally want to know how this number depends on the tissue microgeometry, and how it would change, e.g., if cells shrink or change shape $$$-$$$ e.g., what happens with extra-axonal diffusivity due to demyelination or axonal loss, when the extra-axonal space morphology changes.
The problem of finding $$$D_\infty$$$ as function of geometry has been excruciatingly difficult for any realistic packings of the "building blocks" of a heterogeneous medium. An equivalent problem of finding the dielectric function, or the electrical conductivity, of a suspension of “grains” in a “matrix” goes back to the mid-19th century [14,15], and has been a testing ground for effective medium approaches [3,16-20]. They work well for small volume fractions of the "grains" (in our case, cells) [14-19], or when the properties of the grains and of the matrix are similar [3,20]. For the physiologically relevant tight packing of practically impermeable cells, these approaches fail: the Hashin-Shtrikman bounds [21] and the Bruggeman-Sen solution [18,19] give only order-of-magnitude estimates, with ~100% errors for the physiologically relevant extra-cellular water fraction ~20%. Technically, as each grain (cell) strongly disturbs the diffusion paths, and their mutual effects are important due to a tight packing, there seems no obvious “small parameter” to control the convergence of any perturbative expansion.
The two-dimensional problem was treated analytically and validated numerically [22] for the case of random packing of axons (disks in a cross-section) with a broad distribution of disk sizes, where the approximate analytical solution emphasized a different role of rare large disks, which could be treated as added in an uncorrelated way into a "matrix" of ordered small disks, given that small axons tend to maintain local short-range order more than the rare thick ones. This approximation [22] is not perfect, but generally agrees within a few percent with Monte Carlo simulations, and exhibits nontrivial qualitative features, of very strong sensitivity of the tortuosity to the demyelination (shrinkage of outer diameter for all disks), and a non-monotonic and weakly-varying dependence of $$$D_\infty$$$ on the axonal loss (random removal of the disks). The experimentally obtained trends of the extra-axonal diffusion coefficient transverse to major white matter bundles in the cohorts of multiple sclerosis and Alzheimer's patients agree with the predicted theoretical/numerical behavior for $$$D_\infty$$$ with respect to demyelination and axonal loss [23].
In three dimensions, the tortuosity problem at large cell volume fraction is not solved in a satisfactory way; however, for a practically relevant case of brain tumors [13], where cell fraction $$$f$$$ is quite low, about 0.5, the Bruggeman-like solution [3,18,19] works acceptably, yielding the so-called Archie's law $$$D_\infty = (1-f)^{1/2}D_0$$$. (In two dimensions, the corresponding law would yield $$$D_\infty = (1-f) D_0$$$.) Generally, the higher the spatial dimensionality, the better the mean-field effective-medium approaches work; the two-dimensional Archie's law breaks down for $$$f\gtrsim 0.4$$$. It suffices to say that generally, the tortuosity problem remains unsolved.
Finally, we mention that the functional form of the overall diffusion propagator with the non-Gaussian corrections in the long-time limit was approximately found in ref. [24], relating it to the density-density correlation function of the pore space (in our case, of the extra-cellular space). This solution yields that the non-Gaussian corrections (to the fully Gaussian propagator in the tortuosity limit) decay in an inverse power-law fashion as a function of diffusion time, representing the residual coarse-graining at the large $$$L(t)$$$.
Acknowledgements
Research was sponsored in part by NIH/NINDS grant R01 NS088040References
[1] DS Novikov et al. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. Preprint arXiv:1612.02059 (original: 2016), version 2 (2018)
[2] PP Mitra et al. Short-time behavior of the diffusion coefficient as a geometrical probe of porous media. Phys Rev B 47, 8565 (1993)
[3] LL Latour et al. Time-dependent diffusion of water in a biological model system. PNAS 91 (1994) 1229
[4] DS Novikov and VG Kiselev. Surface-to-volume ratio with oscillating gradients. JMR 210, 141 (2011)
[5] G Lemberskiy et al. Validation of surface‐to‐volume ratio measurements derived from oscillating gradient spin echo on a clinical scanner using anisotropic fiber phantoms. NMR in Biomedicine. 2017, e3708.
[6] O Reynaud et al. Surface-to-Volume Ratio Mapping of Tumor Microstructure Using Oscillating Gradient Diffusion Weighted Imaging. MRM 76, 237-247 (2016)
[7] DS Novikov et al. Revealing mesoscopic structural universality with diffusion. PNAS 111, 5088 (2014)
[8] LM Burcaw, E Fieremans, DS Novikov. Mesoscopic structure of neuronal tracts from time-dependent diffusion. NeuroImage 114 (2015) 18-37
[9] E Fieremans et al. In vivo observation and biophysical interpretation of time-dependent diffusion in human white matter. NeuroImage 129 (2016) 414.
[10] S De Santis et al. Including diffusion time dependence in the extra-axonal space improves in vivo estimates of axonal diameter and density in human white matter. NeuroImage 130 (2016) 91.
[11] H-H Lee, E Fieremans, DS Novikov. What dominates the time dependence of diffusion transverse to axons: Intra- or extra-axonal water? NeuroImage (2017), doi:10.1016/j.neuroimage.2017.12.038
[12] O Reynaud et al. Pulsed and oscillating gradient MRI for assessment of cell size and extracellular space (POMACE) in mouse gliomas. NMR Biomed. 2016; 29, 1350
[13] O Reynaud. Time-Dependent Diffusion MRI in Cancer: Tissue Modeling and Applications. Front. Phys. 5:58 (2017); doi: 10.3389/fphy.2017.00058
[14] OF Mossotti, Mem. di mathem. e fisica in Modena 24 11 (1850) 49.
[15] JC Maxwell, A Treatise on Electricity and Magnetism (Clarendon, 1892).
[16] JCM Garnett, Phil Trans R Soc Lond, B203 (1904) 385.
[17] R Landauer. Electrical conductivity in inhomogeneous media. AIP Conf Proc 40 (1978) 2.
[18] DAG Bruggeman. Berechnzcrrg verschiedcner physikalicher Konstanten von heterogenen Substanzen. Ann Phys (Leipzig) 24 (1935) 636.
[19] PN Sen et al. A self-similar model for sedimentary rocks with application to the dielectric constant of fused glass beads. Geophysics 46 (1981) 781.
[20] DS Novikov & VG Kiselev. Effective medium theory of a diffusion-weighted signal. NMR Biomed 23 (2010) 682.
[21] Z Hashin & S Shtrikman. A variational approach to the theory of effective magnetic permeability of multiphase materials. J Appl Phys 33 (1962) 3125.
[22] DS Novikov and E Fieremans. Relating extracellular diffusivity to cell size distribution and packing density as applied to white matter. Proc. ISMRM 20 (2012), p. 1829
[23] E Fieremans. Diffusion distinguishes between axonal loss and demyelination in brain white matter. Proc. ISMRM 20 (2012), p. 465.
[24] PP Mitra et al. Diffusion propagator as a probe of the structure of porous media. Phys Rev Lets 68, 3555 (1992)
Figures