Principles of Probe Design for Molecular Imagers
1Department of Bioengineering, The University of Tokyo, Tokyo, Japan, 2Group of Quantum-state Controlled MRI, National Institutes for Quantum and Radiological Science and Technology (QST)., Chiba, Japan
Synopsis
Molecular imaging allows the visualization of biological events in real-time at tissue, cellular and subcellular levels in living systems by merging conventional imaging techniques with probes designed to report the expression of biomarkers or variations in physiological factors. Successful molecular imaging agents should provide high contrast intensity with low noise-to-signal ratio at the target in vivo for sufficient time. Herein, I recapitulate the principles for designing effective molecular imaging probes for various imaging modalities, and provide fundamental strategies for the development of these probes and their application in biology, diagnosis and therapy.
Molecular imaging is the visualization, characterization and measurement of biological events at molecular, cellular and tissue levels in vivo [1]. Thus, molecular imaging can be advantageously exploited for the diagnosis and management of diseases, such as cancer and cardiovascular and neurological diseases, providing the possibility for early and precise treatments. A wide range of preclinical and clinical imaging modalities can be used for molecular imaging depending of the purpose of the assessment. Thus, some modalities, e.g. computed tomography (CT), can provide anatomical information of the targeted tissues, while other modalities, such as optical fluorescence microscopy, magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), are useful for obtaining functional information and tracing biological processes [2]. These modalities should be usually combined with specifically designed probes capable of reporting the biochemical information in cells and tissues with sufficient intensity and sensitivity. Thus, molecular imaging probes designed to detect subtle biological processes must be highly sensitive, as they are required to detect small target amounts. Moreover, the probes should not disturb the biological systems, and deliver adequate signal at minimal dosage. The probes should also attain high contrast ratio for trustful imaging, which can be achieved by developing probes with high target-to-background ratio through control of the targeting ability or the clearance of the probes, as well as by designing the probes to become activated after sensing the biological markers. It is important that the probes are stable in vivo, and remain intact during their circulation in the body, reducing their distribution to reticuloendothelial system (RES), for increasing the efficiency of the imaging and avoiding off-target signaling. In addition, the probes should be safe with low immunogenicity and toxicity, and their production should be cheap and scalable, minding their clinical applicability since the initial stages of development.
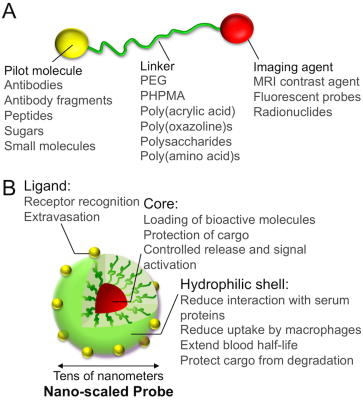
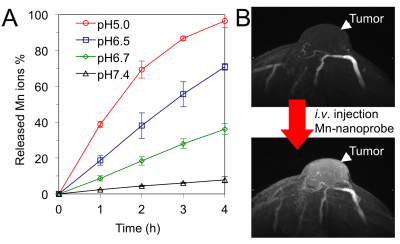
The general features of a molecular imaging probe are illustrated in Figure 1. A basic design consists in a ligand moiety, such as a small molecule, a peptide, a protein or an antibody, and a contrast agent joined by a chemical linker, for example, biocompatible poly(ethylene glycol) (PEG) (Figure 1A). The ligands can bind to particular biomarkers and offer information of various biological processes at the molecular level, with ligands having highly specific binding resulting in lower non-specific distribution to other tissues. Moreover, the linker can be used for controlling the pharmacokinetics of the probes by adjusting its molecular weight, its hydrophilicity, its flexibility, its charge and its interaction with the biological environments. The combination of different contrast agents in the probe can be further exploited for multi-modal imaging techniques. Nano-scaled materials can be also applied for developing molecular imaging probes with unique characteristics (Figure 1B). The compartmentalized nanostructure of these probes allows isolating the contrast agents from the surrounding biological environments, thereby, protecting the imaging agents from inactivation, as well as providing mechanisms for increasing the signal intensity [3], or triggering the contrast ability upon sensing external stimuli. For example, pH-responsive nano-scaled probes loading paramagnetic Mn2+ ions (Figure 2A) showed minimal signal in healthy tissues, but enhanced the contrast of tumors after the triggered release of the Mn2+ ions at the acidic intratumoral regions (Figure 2B) [4]. Moreover, the surface of the nano-scaled probes can be engineered to introduce ligands for tracing particular markers, as well as overcoming biological barriers [5, 6]. The installation of several ligand moieties on the surface of nano-scaled probes results in multivalency effects that can exponentially increase the affinity to biological targets. In addition, the ability for co-loading multiple payloads, including imaging and therapeutic agents, within the same nano-scaled platform can be effectively used for image-guided therapy and seamless diagnosis and therapy, so called theranostics, by exploiting several imaging modalities. It is important to note that tuning the drug/probe ratio is a major challenge in theranostic systems for avoiding toxicities, while maintaining the function of drugs and imaging. Thus, nano-scaled probes can satisfy both precision molecular imaging and effective therapeutic requirements, aiming for unprecedented treatment strategies.
Acknowledgements
JP16H03179 and JP25750172 from JSPS, and 16cm0106202h0001 from AMEDReferences
[1] Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology. 219 (2001) 316–333.
[2] Massoud, T.F.; Gambhir S.S. Molecular Imaging in Living Subjects: Seeing Dundamental Biological Processes in a New Light. Genes Dev. 17 (2003) 545–580.
[3] Kaida, S.; Cabral, H.; Kumagai, M.; Kishimura, A.; Terada, Y.; Sekino, M.; Aoki, I.; Nishiyama, N.; Tani, T.; Kataoka, K. Visible Drug Delivery By Supramolecular Nanocarriers Directing To Single-Platformed Diagnosis And Therapy Of Pancreatic Tumor Model. Cancer Res. 70 (2010) 7031-7041.
[4] Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T; Matsumoto, Y.; Koyama, H.; Cabral H.; et al. Cyclic RGD-Linked Polymeric Micelles For Targeted Delivery Of Platinum Anticancer Drugs To Glioblastoma Through The Blood–Brain Tumor Barrier. ACS Nano 7 (2013) 8583-8592.
[5] Anraku, Y.; Kuwahara, H.; Fukusato, Y.; Mizoguchi, A.; Ishii, T.; Nitta, K.; Matsumoto, Y.; Toh, K.; Miyata, K.; Uchida, S.; et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 8 (2017) 1001.
Figures