Combining fMRI with Advanced Neurotechniques
1Max Planck Inst. for Biological Cybernetics, Tuebingen, Germany, 2Martinos Center, MGH/HMS, Boston, MA, United States
Synopsis
By combining fMRI with fiber optic calcium recording and optogenetics, as well as two-photon microscopy or
Decipher the neuro-glio-vascular contribution to brain state fluctuation with multi-modal fMRI.
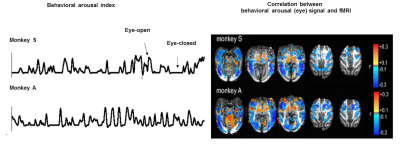
Functional MRI (fMRI) detects brain dynamics based on the neurovascular coupling1-4. Despite its hemodynamic vascular origin5,6, fMRI has been mainly used for brain function and connectivity mapping7-9. Simultaneous fMRI with electrophysiological recordings reveal the neuronal correlate of fMRI signal in both task-related and resting-state conditions 10,11. Besides the “default mode network” mapping with fMRI during the resting-state 12,13, the hemodynamic signal fluctuation has been shown to be closely correlated to the arousal state switches detected by resting-state fMRI (rs-fMRI) and eye open-close arousal index14 (Fig 1). Two intriguing observations highlight the unique spatial dynamic correlation patterns: the brain state fluctuation associated with arousal as eye-opening is correlated with 1). decreased fMRI signals in the whole cortex,and 2). increased fMRI signals in the thalamus.
The anti-correlation of the cortical and subcortical activity (including thalamus), especially at the alpha rhythms has been observed in the wakefulness and sleep transition of vigilance states15-17. However, the neurovascular coupling events underlying the negative cortical fMRI correlation to the arousal state fluctuation remain unclear. To better understand the cellular and vascular contributions to the brain-state dependent fMRI signal fluctuation in the whole-brain mapping scheme, it will help elucidate the subcortical neuromodulatory mechanism of the anti-correlated cortical and subcortical activity.
Multi-modal fMRI platform to map the neuro-glial-vascular (NGV) interactions:
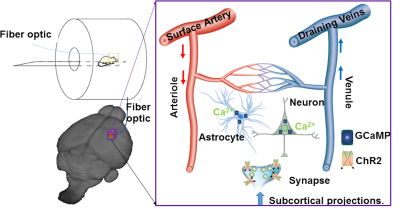
Using Ca2+ sensitive dyes or genetically encoded Ca2+ indicators, the simultaneous fMRI and fiber optic Ca2+ recording have been previously reported in animal brains18-20. The high specificity and fast kinetics of the endogenous indicators make it possible to monitor the dynamic signal from specific cell types in transgenic animals or functional nuclei by viral vector expression21,22. Besides two-photon microscopic (2PM) imaging, fiber optical recordings have been implemented to detect deep brain dynamic signals in animals with fMRI because of its ability to record deep brain activity with less electromagnetic interference in the MR scanner in both anesthetized and awake animals23,24. By merging fMRI with fiber optic Ca2+ recording and optogenetics, we have developed a multi-modal fMRI platform to specify the unique cellular and vascular contributions to fMRI signals, as well as to map the circuit-specific brain activation upon optogenetic activation (Fig 2)23.
Intrinsic astrocytic Ca2+ signaling is associated with the distinct fMRI functional patterns:
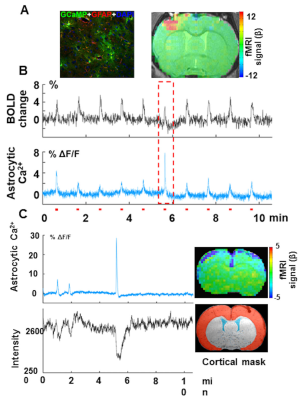
our preliminary data show simultaneous acquisition of evoked astrocytic Ca2+ signal mediated by GCaMP6f and fMRI from the anesthetized rat upon forepaw stimulation (Fig 3A). Besides the evoked astrocytic Ca2+ spikes corresponding to the positive BOLD signal per stimulus train, sporadic astrocytic Ca2+ spikes with high amplitude were detected in coincidence with the evoked BOLD signal with reduced amplitude (Fig 3B, red box). Interestingly, this intrinsic astrocytic Ca2+ signal has also been detected in rats at rest, concurrently with the negative BOLD signal through the whole cortex (Fig 3B). Thus, we have observed that:
1). the normally evoked astrocytic Ca2+ signal is coupled to the positive BOLD signal in the activated cortical regions; in contrast,
2). the intrinsic astrocytic Ca2+ signal is coupled to the negative BOLD signal detected in the whole cortex.
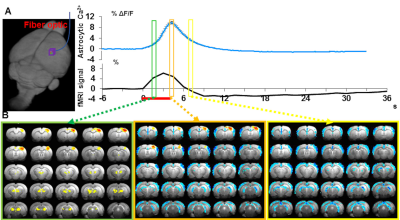
The two distinct gliovascular coupling events could occur concurrently, showing evoked and intrinsic astrocytic Ca2+ signals together upon stimulation. Our preliminary data (Fig 4) show the concurrent event-related whole-brain functional maps with similar spatial correlation patterns to that of the rs-fMRI correlation to the arousal state fluctuation in the unanesthetized monkey14,24:
1). the positive BOLD signal in the central thalamus and midbrain reticular formation, followed by 2). the negative BOLD signal in the whole cortex.
These results indicate a potential gliovascular interaction model specifically relevant to the whole brain rs-fMRI signal fluctuation, which correlates to the brain state changes underlying arousal switches.
In summary, by combining fMRI with the advanced neurotechniques, we are able to decipher the detailed molecular and cellular contributions to the fMRI signal at varied brain states in both normal and physiological conditions of both animal and human brains.
Acknowledgements
We thank Duyn J and Chang C to contribute figures in this abstract. The other part of this work was supported by DFG SPP-1655 and the internal funding from Max Planck Society for X. Y. and HHMI support for T. S. We thank Dr. Pohmann, R.; Dr. Merkle H., and Buckenmaier, K. for technical support. We thank Ms. Schulz, H. and Fischer, S. for animal maintenance support. We thank the AFNI team for the software support. We thank Dr. Khakh BS, and GENIE Program and the Janelia Farm Research Campus to provide the astrocyte-specific promoter and GCaMP6 plasmids.References
1 Ogawa, S. et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences of the United States of America 89, 5951-5955 (1992).
2 Kwong, K. K. et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America 89, 5675-5679 (1992).
3 Bandettini, P. A., Wong, E. C., Hinks, R. S., Tikofsky, R. S. & Hyde, J. S. Time course EPI of human brain function during task activation. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 25, 390-397 (1992).
4 Belliveau, J. W. et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254, 716-719 (1991).
5 Ogawa, S., Lee, T. M., Kay, A. R. & Tank, D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America 87, 9868-9872 (1990).
6 Rosen, B. R., Belliveau, J. W., Vevea, J. M. & Brady, T. J. Perfusion imaging with NMR contrast agents. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 14, 249-265 (1990).
7 Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869-878, doi:10.1038/nature06976 (2008).
8 Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. Neuroscience 8, 700-711, doi:10.1038/nrn2201 (2007). 9 Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 34, 537-541 (1995).
10 Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150-157, doi:10.1038/35084005 (2001).
11 Scholvinck, M. L., Maier, A., Ye, F. Q., Duyn, J. H. & Leopold, D. A. Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences of the United States of America 107, 10238-10243, doi:10.1073/pnas.0913110107 (2010).
12 Raichle, M. E. et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America 98, 676-682, doi:10.1073/pnas.98.2.676 (2001).
13 Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America 100, 253-258, doi:10.1073/pnas.0135058100 (2003).
14 Chang, C. et al. Tracking brain arousal fluctuations with fMRI. Proceedings of the National Academy of Sciences of the United States of America 113, 4518-4523, doi:10.1073/pnas.1520613113 (2016).
15 Olbrich, S. et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. NeuroImage 45, 319-332, doi:10.1016/j.neuroimage.2008.11.014 (2009).
16 Goldman, R. I., Stern, J. M., Engel, J., Jr. & Cohen, M. S. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13, 2487-2492, doi:10.1097/01.wnr.0000047685.08940.d0 (2002).
17 Ong, J. L. et al. Co-activated yet disconnected-Neural correlates of eye closures when trying to stay awake. NeuroImage 118, 553-562, doi:10.1016/j.neuroimage.2015.03.085 (2015).
18 Schulz, K. et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nature methods 9, 597-602, doi:10.1038/nmeth.2013 (2012).
19 Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature methods 6, 875-881, doi:10.1038/nmeth.1398 (2009).
20 Schwalm, M. et al. Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. Elife 6, doi:10.7554/eLife.27602 (2017).
21 Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Frontiers in molecular neuroscience 6, 2, doi:10.3389/fnmol.2013.00002 (2013).
22 Kim, C. K. et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nature methods 13, 325-328, doi:10.1038/nmeth.3770 (2016).
23 Yu, X. in Small Animal Imaging Basics and Practical Guide (eds F. Kiessling, B Pichler, & P. Hauff) (Springer International Publishing, 2017).
24 Wang, M., He, Y., Sejnowski, T. J. & Yu, X. Brain-state dependent astrocytic Ca(2+) signals are coupled to both positive and negative BOLD-fMRI signals. Proceedings of the National Academy of Sciences of the United States of America 115, E1647-E1656, doi:10.1073/pnas.1711692115 (2018).
Figures