What Are We Really Measuring in CEST?
1Vanderbilt University Medical Center, United States
Synopsis
This talk will examine the underlying drivers of the CEST signal, and it will discuss the implications for choosing CEST pulse sequences and metrics that maximize solute specificity.
Target audience
The target audience is anyone interested in understanding CEST specificity. The only background required is viewing the previous CEST talk (by Jerschow).Outcome/Objectives
The goal is to understand the connection between the CEST parameters (such as the solute concentration and exchange rate) and the measured CEST signal and calculated CEST metric. This relationship dictates the resulting solute specificity, which is the ultimate goal of CEST imaging.Discussion
Most MRI contrast is generated by exchange phenomenon. While relaxation theory is typically taught using the ideas of Bloembergen, Purcell, and Pound (BPP) and focusing on the effects of molecular motion, it is magnetization exchange that is often the essential contributor to relaxation effects in vivo, especially at higher field strengths. In this sense, simple T1- and T2-weighted imaging are measures of magnetization exchange. However, these commonly applied imaging methods generate non-specific contrast, since many distinct exchanging and non-exchanging phenomenon contribute.
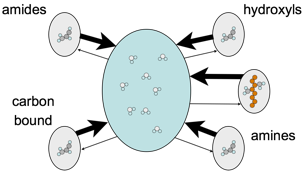
Magnetization transfer (MT) was developed as a means for imaging a more specific biophysical phenomenon: exchange between water and macromolecules. MT in turn led to chemical exchange saturation transfer (CEST), a means for generating contrast based on magnetization exchange with particular sites, as illustrated in the figure, which reside on particular molecules. However, the achievable specificity in a CEST experiment is difficult to assess. The signal is a complex function of the tissue and acquisition parameters. This talk will explore this dependency. How much do T1and MT changes affect the CEST signal? Can CEST signals be combined to generate metrics that are specific to a particular solute parameter, such as concentration? How do you design such a metric, and how good are the current published metrics?
To answer these questions, the talk is broken into five parts: The model, the equations, simulations of signal dependencies, metrics, analytic solutions, and novel sequences and the quest for specificity. These are interrelated issues. For each sequence, there are corresponding metrics, designed to maximize solute specificity or to quantify a particular tissue parameter. This method/metric design is typically informed by an analytic signal approximation expressed in terms of the underlying tissue parameters. And this entire combination of method, metric, specificity, and analytic approximation is in the service of a particular application, targeting a particular solute, frequency offset, and exchange rate. In this talk, we will briefly touch on these issues to lay the groundwork for the rest of the session.