MRI Capable Theranostic Agents
1Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
New generation magnetic resonance imaging (MRI) contrast agents such as nanoparticles can be developed as theranostic agents which integrate the MRI contrast enhancing capability with therapeutic functions. The objective of this lecture is to give the audience an update of the recent developments in MRI enabled theranostics and stimulate the interests and new ideas to further advance the research and clinical translations of MRI enabled theranostics. The rational design of MRI capable theranostics agents, approaches for making the desired theranostic platforms, and MRI methods developed to enable MRI visualization of delivery processes will be discussed and presented with examples.
Theranostics & Novel Molecular Probes
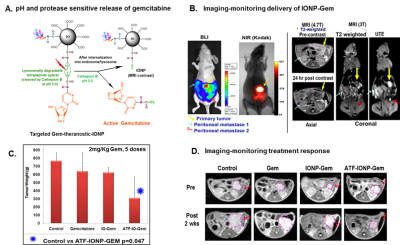
Various classes of new generation magnetic resonance imaging (MRI) contrast agents such as nanoparticles have potential to be developed as theranostic agents which integrate the MRI contrast enhancing capability with therapeutic functions [1-2]. These theranostic MRI contrast agents can be used for targeted delivery of therapeutics while using MRI to track, or even quantify, the distribution, accumulation and clearance of the agents noninvasively in preclinical animal models or patients, and subsequently to monitor the treatment responses [3-4]. With MRI widely available and typical need of relatively large dosages of low toxicity MRI contrast agents, well-engineered MRI based theranostics has advantages in targeting, improved pharmacokinetics and more importantly allowing for “seeing and believing” the delivery of the agents. On the other hand, MRI based theranostics has expanded MRI from traditional diagnostic applications to image-guided therapy applications, increasing the value of MRI in healthcare research and delivery. The emerging field of theranostics is highly interdisciplinary and attracts a wide range of scientific and engineering expertise, including chemistry, biomaterials, pharmacology, imaging sciences and technology, as well as clinical specialties that can apply image-guided treatments. To those who are interested in working in the field, this lecture attempts to introduce the general concept and rational design of the theranostics agents, chemical and biological properties needed in a theranostics agent, strategies for properly loading and releasing the therapeutics, approaches for delivery of theranostics and some examples of MRI capable theranostic platforms (shown in Figure) that have been developed for MRI and drug delivery[5-6], specifically superparamagnetic iron oxide nanoparticles (SPIO or IONP) which have been used in clinical applications. Complementary to the material development, MRI methods that have been developed and applied to enable MRI contrast enhancement and visualization of delivery processes in vivo will be presented. The lecture will also discuss current challenges in developing and applying theranostics platform, for example, delivery efficiency, molecular targeting, image quantification, and biocompatibility and clearance of theranostics agents [7-8]. The objective of this lecture is to give the audience an update of the recent developments in MRI capable theranostics and stimulate the interests and new ideas to further advance the research and clinical translations of theranostics that is facilitated by the power of MRI technology.Acknowledgements
No acknowledgement found.References
1. Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011; 44(10):842-52.
2. Jokerst JV, Gambhir SS. Molecular imaging with theranostics nanoparticles. Acc Chem Res. 2011; 44(10):1050-60.
3. Penet MF, Jin J, Chen Z, Bhujwalla ZM. Magnetic Resonance Imaging and Spectroscopy in Cancer Theranostic Imaging. Top Magn Reson Imaging. 2016; 25(5):215-221.
4. Li Y, Chen H, Xu J, Yadav NN, Chan KW, Luo L, McMahon MT, Vogelstein B, van Zijl PC, Zhou S, Liu G. CEST theranostics: label-free MR imaging of anticancer drugs. Oncotarget. 2016; 7(6):6369-784:
5. Li K, Nejadnik H, Daldrup-Link HE. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov Today. 2017; 22(9):1421-1429.
6. Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, Nie S, Mao H, Yang L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013; 7(3):2078-89.
7. Wang L, Huang J, Chen H, Wu H, Xu Y, Li Y, Yi H, Wang YA, Yang L, Mao H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1-T2 switchable magnetic resonance imaging contrast. ACS Nano. 2017; 11(5):4582-4592.
8. Hu Y, Mignani S, Majoral JP, Shen M, Shi X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem Soc Rev. 2018; 47(5):1874-1900.
Figures