Imaging of Cardiac Microstructure
1Institute for Biomedical Engineering, University and ETH Zurich, Switzerland
Synopsis
Microstructural imaging in the beating heart by means of diffusion weighted imaging suffers from cardiac motion induces signal dephasing. Different approach to address cardiac motion by MR sequence design and image post-processing are reviewed. Potentials and limitations are discussed and cases in which different approaches fail illustrate.
Introduction
Unlike skeletal muscle, the heart muscle does not contract between two fixed points. Its mechanics is hence directly connected to its rather complicated structure. Alterations in microstructure have been reported for myocardial infarction (1,2), hypertrophic (3) and dilated cardiomyopathies (4), valvular diseases (5) and have been linked to abnormal heart function. Over the past decades, MRI has emerged as versatile tool to probe microstructure in various organs non-invasively. When talking about microstructural imaging by means of diffusion tensor imaging, q-ball imaging, diffusion spectrum imaging or even the assessment of organ perfusion by intra voxel incoherent motion, common ground is found by diffusion weighted imaging: a method to sensitize the applied MR-sequence to random motion of water molecules. Diffusion sensitizing by pulsed field gradients dates back to 1965 when Stejskal and Tanner incorporated a pair of field gradients into a spin echo sequence (6). Ever since, diffusion weighting has been applied to almost every organ in the human body. Focussing on the heart, however, it took another 29 years to conduct diffusion weighting successfully in an MRI experiment (7). The faced challenge is obvious: How can we measure molecular water displacement in the micrometre range inside an organ that moves on the order of centimetres? The aim of this educational is to review current approaches to generate diffusion contrast in the beating heart, compare their potentials as well as limitations and illustrate cases in which different approaches fail.Diffusion encoding in the beating heart
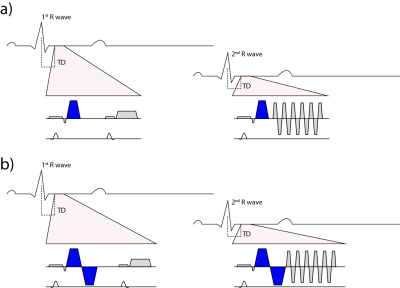
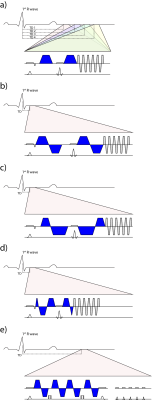
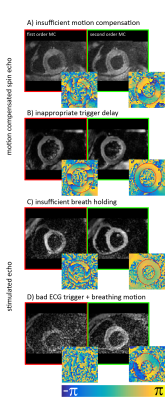
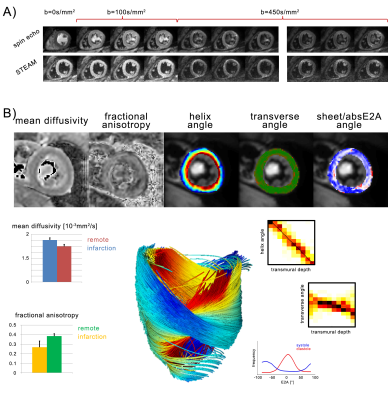
To address the challenge of cardiac motion in a diffusion weighted imaging experiment, one needs to design a filter sensitive to a random walk, but insensitive to myocardial motion. This can be achieved by a dedicated cardiac MR (CMR) sequence design during acquisition or during post processing. Figure 1 & 2 illustrate current approaches. The first attempts to generate diffusion contrast in the beating heart was based on a Stimulated Echo Acquisition Mode (STEAM) sequence (7,8). This approach relies on splitting the modulation and demodulation part of a STEAM sequences across two consecutive heart beats (figure 1a). By doing so, periodic motion such as cardiac contraction is filtered from random motion of water diffusion. It is therefore crucial, that the periodic constraint is valid, meaning that each material point follows the same trajectory during two consecutive heart beats. A mismatch in motion states will inevitably lead to signal dephasing (9). By splitting diffusion weighting across one R-R interval, the diffusing water molecules are given sufficient time to probe their environment, but also to change compartments by transmembrane exchange. Early on, cyclic deformation, i.e. myocardial strain, has been identified as potential source of diffusion measurement bias (8,9). Approaches to address these measurement errors included optimal sequences timing during so called “strain sweet spot” (10), the use of bipolar gradients (11) (Figure 1b) or additional strain measurements while treating the myocardium as a continuum (12). Recent measurements in in-vivo and arrested porcine hearts suggest, that a more complex model may be required for strain correction decomposing the deformation gradient field into a rotational and a stretch component at the myocyte and cleavage plane level (13). A more SNR efficient alternative to STEAM (14) is based on Spin echo sequences. Since periodic motion of consecutive heart beats cannot be utilized here, a different “filter” is required. It is necessary to examine phase evolution of magnetization moving through a temporal varying gradient waveform. The Taylor expansion of the motion trajectory comes in handy by parameterizing the water molecule’s phase by gradient moments. A conventional Stejskal Tanner encoding scheme rephases stationary magnetization by nulling the zeroth gradient moment. To apply such an encoding scheme to cardiac imaging, stationary tissue is required, making the quiescence period during diastole a potential candidate for imaging. By sweeping the trigger delay across a time interval in diastole, maximum intensity projection (MIP) may be applied to filter motion induced signal voids (15) (Figure 2a). This approach requires image denoising to avoid signal bias due to the applied MIP (16,17). It is based on the assumption, that for each pixel at least one time point during the acquisition window exists, where the myocardium is still. To make use of averaging multiple acquisitions without MIP, it is necessary to translate the filter effect from post processing to acquisition. Nulling higher order moments (18–21) allows for imaging during contractile motion without cardiac motion induced signal dephasing (Figure 2b-c). E.g. in second order motion compensated diffusion encoding, material points have to follow a motion trajectory that is defined by an initial position, velocity and acceleration. Any deviation from such a trajectory, e.g. due to jerk components, during the application of diffusion encoding gradients, will lead to phase accumulation and potential signal dephasing. The validity of this assumption has been confirmed in in-vivo rat experiments (21) as well as comparison of in-vivo with ex-vivo imaging (22). It is pivotal to keep the waveform duration of motion compensated diffusion gradients as short as possible to reduce motion sensitivity and echo time. This can be achieved by applying convex optimization during waveform construction (23) (Figure 2 d) and the use of high performance hardware. It is noted, that for all previously mentioned techniques, diffusion encoding has been incorporated into an imaging sequences. An alternative approach has been proposed separating the sequence into a diffusion sensitizing module and an imaging module (24) (Figure 2e). While similar assumption hold with respect to diffusion encoding as for the previously discussed SE technique, this sequence allows to choose the form of readout to address shortcomings of long spiral or echo planar imaging readouts. With the techniques presented above it is important to know what prerequisites have to be met and how measurement errors appear on the resulting image. Signal attenuation due to physiological motion may be mistaken for diffusion contrast. To better discriminate the source of signal attenuation, it is worthwhile to investigate the image phase. Figure 3 illustrates examples at which the diffusion imaging constrains have been violated. Having mastered diffusion weighted imaging, one can now derive parameters characterizing tissue properties. Due to the limited amount of time in an in-vivo setting, diffusion tensor imaging (25) is commonly employed, demanding a certain diffusion weighting and number of diffusion encoding directions (26). From the diffusion tensor, scalar parameters can be derived such as the mean diffusivity, fractional anisotropy and shape coefficients. Additionally, the principal myofiber orientation and sheetlet alignment (Figure 4) can be computed. It is important to know, that these parameters have a dependency on the imaging technique employed (27,28).Conclusion
Currently, STEAM and motion compensated spin echo techniques are most frequently used when generating diffusion contrast in the beating heart. However, it is important to be familiar with the prerequisites, limitations and differences of each sequence. To interpret diffusion measurement results appropriately, it is key to understand how measurement errors arise and how they manifest in the acquired images.Acknowledgements
No acknowledgement found.References
1. Sosnovik DE et al. Diffusion spectrum MRI tractography reveals the presence of a complex network of residual myofibers in infarcted myocardium. Circ. Cardiovasc. Imaging 2009;2:206–12.
2. Wu Y et al. Diffusion tensor MRI study of myocardium structural remodeling after infarction in porcine model. Conf Proc IEEE Eng Med Biol Soc 2006;1:1069–1072.
3. Ferreira PF et al. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014;16:87
4. Von Deuster C et al. Studying Dynamic Myofiber Aggregate Reorientation in Dilated Cardiomyopathy Using in Vivo Magnetic Resonance Diffusion Tensor Imaging. Circ. Cardiovasc. Imaging 2016;9.
5. Mcclymont D et al. Evaluation of non-Gaussian diffusion in cardiac MRI. Magn. Reson. Med. 2016;0. doi: 10.1002/mrm.26466.
6. Stejskal EO, Tanner JE. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965;42:288.
7. Edelman RR et al. In vivo measurement of water diffusion in the human heart. Magn. Reson. Med. 1994;32:423–8.
8. Reese TG et al. Imaging myocardial fiber architecture in vivo with magnetic resonance. Magn. Reson. Med. 1995;34:786–91.
9. Reese TG et al. Measuring Diffusion in the Presence of Material Strain. J. Magn. Reson. Ser. B 1996;112:253–258. 10. Tseng W, Reese T. Cardiac diffusion tensor MRI in vivo without strain correction. Magn. Reson. Med. 1999;403:393–403.
11. Dou J et al. Cardiac diffusion MRI without motion effects. Magn. Reson. Med. 2002;48:105–114. doi: 10.1002/mrm.10188.
12. Stoeck CT et al. Dual-phase cardiac diffusion tensor imaging with strain correction. PLoS One 2014;9. doi: 10.1371/journal.pone.0107159.
13. Ferreira PF et al. Evaluation of the Impact of Strain Correction on the Orientation of Cardiac Diffusion Tensors With In Vivo and Ex Vivo Porcine Hearts. 2017;0. doi: 10.1002/mrm.26850.
14. von Deuster C et al. Spin echo versus stimulated echo diffusion tensor imaging of the in vivo human heart. Magn. Reson. Med. 2016;76:862–872. doi: 10.1002/mrm.25998.
15. Rapacchi S et al. Low b-Value Diffusion-Weighted Cardiac Magnetic Resonance Imaging: Initial Results in Humans Using an Optimal Time-Windiw Imaging Approach. 2012;46:751–758.
16. Pai VM et al. PCATMIP: enhancing signal intensity in diffusion-weighted magnetic resonance imaging. Magn. Reson. Med. 2011;65:1611–1619.
17. Wei H et al. Free-breathing diffusion tensor imaging and tractography of the human heart in healthy volunteers using wavelet-based image fusion. IEEE Trans. Med. Imaging 2015;34:306–316.
18. Pipe JG et al. A progressive gradient moment nulling design technique. Magn. Reson. Med. 1991;19:175–9.
19. Gamper U et al. Diffusion imaging of the in vivo heart using spin echoes--considerations on bulk motion sensitivity. Magn. Reson. Med. 2007;57:331–7.
20. Stoeck CT et al. Second-order motion-compensated spin echo diffusion tensor imaging of the human heart. Magn. Reson. Med. 2015;0
21. Welsh C et al. Higher-Order Motion-Compensation for In Vivo Cardiac Diffusion Tensor Imaging in Rats. IEEE Trans. Med. Imaging 2015;62:1–1.
22. Stoeck CT et al. Direct comparison of in vivo versus postmortem second-order motion-compensated cardiac diffusion tensor imaging. Magn. Reson. Med. 2017;0.
23. Aliotta E et al. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI. Magn. Reson. Med. 2016;0
24. Nguyen C et al. In vivo three-dimensional high resolution cardiac diffusion-weighted MRI: A motion compensated diffusion-prepared balanced steady-state free precession approach. Magn. Reson. Med. 2013;0.
25. Basser PJ et al. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–67.
26. Froeling M et al. DTI of human skeletal muscle: the effects of diffusion encoding parameters, signal-to-noise ratio and T2 on tensor indices and fiber tracts. NMR Biomed. 2013;26:1339–52.
27. von Deuster C et al. Spin echo versus stimulated echo diffusion tensor imaging of the in vivo human heart. Magn. Reson. Med. 2016;76.
28. Scott AD et al. An in-vivo comparison of stimulated-echo and motion compensated spin-echo sequences for 3 T diffusion tensor cardiovascular magnetic resonance at multiple cardiac phases. J. Cardiovasc. Magn. Reson. 2018;20:1–15.
Figures