Looking from Within: Diffusion of Compartment Specific Metabolites
1CEA-MIRCen, France
Synopsis
Diffusion-weighted NMR spectroscopy (DW-MRS) offers the unique ability to non-invasively quantify diffusion properties of endogenous brain metabolites in vivo. In contrast to water molecules, which are ubiquitous in biological tissues, most brain metabolites are confined into the intracellular space, and some of them are even thought to exhibit preferential cellular compartmentation (within neurons or glial cells). It is thus expected that DW-MRS may provide specific cellular information. Here we will see how DW-MRS can be related to cellular microstructure, opening perspectives for non-invasive and quantitative measurements of cell-specific morphology under normal and pathological conditions.
Introduction
Diffusion-weighted NMR spectroscopy (DW-MRS) offers the unique ability to non-invasively quantify the diffusion of endogenous brain metabolites in vivo (1-3). In contrast to water molecules, which are ubiquitous in biological tissues, most brain metabolites are confined into the intracellular space. Their diffusion properties are thus expected to mostly depend on intracellular parameters such as cytosol viscosity, molecular crowding, size and shape of the cell… Furthermore, some metabolites are even thought to exhibit preferential cellular compartmentation: it is generally accepted that N-acetyl-aspartate (NAA) and glutamate (Glu) reside essentially in neurons, whereas myo-inositol (Ins) and choline compounds (tCho) have been reported to be preferentially compartmentalized in glial cells (4). DW-MRS may therefore provide specific information about the intracellular space. Here we will focus on brain applications; however other organs, in particular muscles, have also been studied by DW-MRS.Detecting brain cellular alterations with DW-MRS: an overview
Cellular specificity has been the main motivation driving methodological research and applications of DW-MRS in vivo over the last 25 years. Alterations of metabolite diffusion have been reported in brain diseases, illustrating the potential of the method. DW-MRS has been first applied to ischemic stroke, to elucidate the possible intracellular origin of apparent diffusion coefficient (ADC) decrease observed in DW-MRI. All studies reported a 20-50% decrease of metabolite ADC (5-10), supporting the idea that massive intracellular alterations occurred during ischemic stroke, although the nature of these alterations was not identified by these studies. Metabolite ADC has also been reported to decrease in human healthy aging (10). Some increase of metabolite ADC has been measured in tumors (11,12), but this remains ambiguous as another study reported no change (13). More recently, DW-MRS has been applied in autoimmune diseases and showed promising results in terms of specificity: in multiple sclerosis, NAA ADC decreased along axons in white matter, while water ADC increased (14), suggesting specific intra-axonal alteration; and in systemic lupus erythematosus, increased ADC has been measured in white matter for total creatine and tCho, while NAA exhibited no change, suggesting specific glial alteration (15). Altogether these studies strongly support the unique capabilities of DW-MRS to detect cellular alterations. However, although ADC variations may provide new and specific imaging biomarkers of brain diseases, the fundamental understanding of the origin of these variations remain unclear. The basic reason for this limitation is that many potential factors may affect the ADC, such as cytosol viscosity but also subcellular compartmentation or the size of the cellular compartment, the relative contribution of these various factors being not clearly understood.Interpreting DW-MRS in terms of cellular microstructure
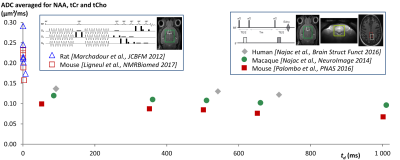
Brain metabolite diffusion is characteristic of diffusion in long and thin cylinders: To try to elucidate what governs metabolite diffusion, our group has engaged in the exploration of very different diffusion times td (from ~1 ms using oscillating gradients (16,17), to 1 sec using stimulated echo (18,19)), to probe brain metabolite diffusion at very different spatial scales. Measurements of ADC as a function of td at short-time scales reveal rapidly decreasing ADC as td is increased, followed by a relatively stable plateau for long td, which is very clear signature of metabolites being “freely” diffusing along long and thin fibers (including in grey matter) (Figure 1). Intracellular viscosity is found to be not much larger than pure water viscosity, as already suggested by previous diffusion studies. Moreover, these measurements allow ruling out any significant contribution of active transport or compartmentation in subcellular structure (such as organelles) to metabolite diffusion. This framework gives solid grounds for modeling DW-MRS in fibers (20), which we also show to account for the non-monoexponential attenuation at high diffusion-weightings (21) (not only for NAA, as already performed in past studies (22,23), but also for other metabolites). This view of diffusion in cylinders is in line with recent double-diffusion-encoding DW-MRS works reporting marked compartment shape anisotropy for brain metabolites (24,25).
Beyond the "long cylinder" model: The long and thin cylinder framework can also lay the foundation for elaborating new models to extract of cell-specific morphological parameters, e.g. as recently proposed for data at ultra-long td (up to 2 sec), where fibers are now considered to exhibit some branching and finite length (26). It appears that reconstructed compartments of supposedly neuronal (NAA, Glu) and glial (Ins, tCho) metabolites are indeed found to be different, with reconstructed glial cells being smaller and less complex than neurons. This strongly supports the view of metabolites essentially diffusing in fibrous structures, and suggests that adequate DW-MRS acquisition and modeling may allow distinguishing and quantifying neuronal and glial morphology. The most recent developments also suggest that models of fibers viewed as smooth cylinder might be refined by introducing small protrusions, such as dendritic spines or astrocytic leaflets, which may account more finely for experimental data (27).
Microstructural alterations probed by DW-MRS: Finally, we evaluate the potential of some of these approaches to quantitatively assess morphological variations under “pathological” conditions, in a mouse model of astrocytic activation. Increased fiber diameter and fiber length are measured for myo-inositol compartment by DW-MRS, consistently with astrocytic hypertrophy as quantitatively assessed by confocal microscopy. Very interestingly, in this model, the diffusion of lactate exhibits very strong variation, suggesting massive remodeling of energy metabolism compartmentation, with lactate shifting from a predominantly astrocytic to a predominantly neuronal compartment.
Conclusion
In conclusion, DW-MRS has progressively emerged as a tool to specifically probe cellular alterations in various neurological disorders. In parallel, the basic understanding of the main features governing metabolite diffusion has progressed, allowing more relevant interpretation of DW-MRS under normal and pathological conditions, opening new possibilities for microstructure quantification, and maybe even yielding new insights into metabolic compartmentation and neuron-astrocyte interactions.Acknowledgements
JV is recipient of grant from the European Research Council about diffusion-weighted MRS (grant agreement No. 336331 – INCELL project).
References
1. Nicolay K, Braun KP, Graaf RA, Dijkhuizen RM, Kruiskamp MJ. Diffusion NMR spectroscopy. NMR in biomedicine 2001;14(2):94-111.
2. Ronen I, Valette J. Diffusion-weighted magnetic resonance spectroscopy. eMagRes 2015;4:733–750.
3. Cao P, Wu EX. In vivo diffusion MRS investigation of non-water molecules in biological tissues. NMR in biomedicine 2016.
4. Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR in biomedicine 2007;20(3):216-237.
5. Wick M, Nagatomo Y, Prielmeier F, Frahm J. Alteration of intracellular metabolite diffusion in rat brain in vivo during ischemia and reperfusion. Stroke 1995;26(10):1930-1933; discussion 1934.
6. van der Toorn A, Dijkhuizen RM, Tulleken CA, Nicolay K. Diffusion of metabolites in normal and ischemic rat brain measured by localized 1H MRS. Magnetic resonance in medicine 1996;36(6):914-922. 7. Dijkhuizen RM, de Graaf RA, Tulleken KA, Nicolay K. Changes in the diffusion of water and intracellular metabolites after excitotoxic injury and global ischemia in neonatal rat brain. Journal of cerebral blood flow and metabolism 1999;19(3):341-349.
8. Abe O, Okubo T, Hayashi N, Saito N, Iriguchi N, Shirouzu I, Kojima Y, Masumoto T, Ohtomo K, Sasaki Y. Temporal changes of the apparent diffusion coefficients of water and metabolites in rats with hemispheric infarction: experimental study of transhemispheric diaschisis in the contralateral hemisphere at 7 tesla. Journal of cerebral blood flow and metabolism 2000;20(4):726-735.
9. Dreher W, Busch E, Leibfritz D. Changes in apparent diffusion coefficients of metabolites in rat brain after middle cerebral artery occlusion measured by proton magnetic resonance spectroscopy. Magnetic resonance in medicine 2001;45(3):383-389.
10. Zheng DD, Liu ZH, Fang J, Wang XY, Zhang J. The effect of age and cerebral ischemia on diffusion-weighted proton MR spectroscopy of the human brain. AJNR Am J Neuroradiol 2012;33(3):563-568.
11. Harada M, Uno M, Hong F, Hisaoka S, Nishitani H, Matsuda T. Diffusion-weighted in vivo localized proton MR spectroscopy of human cerebral ischemia and tumor. NMR in biomedicine 2002;15(1):69-74.
12. Valette J, Giraudeau C, Marchadour C, Djemai B, Geffroy F, Ghaly MA, Le Bihan D, Hantraye P, Lebon V, Lethimonnier F. A new sequence for single-shot diffusion-weighted NMR spectroscopy by the trace of the diffusion tensor. Magnetic resonance in medicine 2012.
13. Hakumaki JM, Poptani H, Puumalainen AM, Loimas S, Paljarvi LA, Yla-Herttuala S, Kauppinen RA. Quantitative 1H nuclear magnetic resonance diffusion spectroscopy of BT4C rat glioma during thymidine kinase-mediated gene therapy in vivo: identification of apoptotic response. Cancer Res 1998;58(17):3791-3799.
14. Wood ET, Ronen I, Techawiboonwong A, Jones CK, Barker PB, Calabresi P, Harrison D, Reich DS. Investigating axonal damage in multiple sclerosis by diffusion tensor spectroscopy. The Journal of neuroscience 2012;32(19):6665-6669.
15. Ercan E, Magro-Checa C, Valabregue R, Branzoli F, Wood ET, Steup-Beekman GM, Webb AG, Huizinga TW, van Buchem MA, Ronen I. Glial and axonal changes in systemic lupus erythematosus measured with diffusion of intracellular metabolites. Brain 2016;139(Pt 5):1447-1457.
16. Marchadour C, Brouillet E, Hantraye P, Lebon V, Valette J. Anomalous diffusion of brain metabolites evidenced by diffusion-weighted magnetic resonance spectroscopy in vivo. Journal of cerebral blood flow and metabolism 2012;32(12):2153-2160.
17. Ligneul C, Valette J. Probing metabolite diffusion at ultra-short time scales in the mouse brain using optimized oscillating gradients and "short"-echo-time diffusion-weighted MRS. NMR in biomedicine 2017;30(1).
18. Najac C, Marchadour C, Guillermier M, Houitte D, Slavov V, Brouillet E, Hantraye P, Lebon V, Valette J. Intracellular metabolites in the primate brain are primarily localized in long fibers rather than in cell bodies, as shown by diffusion-weighted magnetic resonance spectroscopy. NeuroImage 2014;90:374-380.
19. Najac C, Branzoli F, Ronen I, Valette J. Brain intracellular metabolites are freely diffusing along cell fibers in grey and white matter, as measured by diffusion-weighted MR spectroscopy in the human brain at 7 T. Brain structure & function 2016;221(3):1245-1254.
20. Valette J, Ligneul C, Marchadour C, Najac C, Palombo M. Brain Metabolite Diffusion from Ultra-Short to Ultra-Long Time Scales: What Do We Learn, Where Should We Go? Frontiers in Neuroscience 2018;12:2.
21. Palombo M, Ligneul C, Valette J. Modeling diffusion of intracellular metabolites in the mouse brain up to very high diffusion-weighting: Diffusion in long fibers (almost) accounts for non-monoexponential attenuation. Magnetic resonance in medicine 2016.
22. Kroenke CD, Ackerman JJ, Yablonskiy DA. On the nature of the NAA diffusion attenuated MR signal in the central nervous system. Magnetic resonance in medicine 2004;52(5):1052-1059.
23. Ronen I, Ercan E, Webb A. Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. Frontiers in integrative neuroscience 2013;7:13. 24. Shemesh N, Rosenberg JT, Dumez J-N, Muniz JA, Grant SC, Frydman L. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat Commun 2014;5.
25. Shemesh N, Rosenberg JT, Dumez JN, Grant SC, Frydman L. Distinguishing neuronal from astrocytic subcellular microstructures using in vivo Double Diffusion Encoded H-1 MRS at 21.1 T. PloS one 2017;12(10).
26. Palombo M, Ligneul C, Najac C, Le Douce J, Flament J, Escartin C, Hantraye P, Brouillet E, Bonvento G, Valette J. New paradigm to assess brain cell morphology by diffusion-weighted MR spectroscopy in vivo. Proceedings of the National Academy of Sciences of the United States of America 2016;113(24):6671-6676.
27. Palombo M, Ligneul C, Hernandez-Garzon E, Valette J. Can we detect the effect of spines and leaflets on the diffusion of brain intracellular metabolites? NeuroImage 2017.
Figures