Using Tractography to Guide Tumor Resections
1Oxford University FMRIB Centre, United Kingdom
Synopsis
· Tractography remains the only tool available to measure fibre tracts in the living human brain.
· By identifying relationships between tumour and behaviourally essential tracts, tractography offers a clinical tool to predict and minimize risks of neurosurgery.
· Tractography offers benefits in treatment decision making, predicting the extent of tumour removal possible, planning safe access routes and monitoring performance outcomes.
· Application of tractography in neurosurgery relies on detailed knowledge of anatomy and the effects of different acquisitions, analysis methods and pathological confounds on tractography success.
· Standardized methods are much needed to assess the impact of tractography in optimising post-surgical quality of life.
PURPOSE
For intrinsic brain tumours, growing data suggest that greater surgical removal is associated with greater oncological benefit (1, 2). On the other hand, the impact of surgery on white matter fibre bundles is an increasingly important consideration for the quality of the extra lifetime gained by tumour surgery (3). This talk will describe the impact of tractography - as the only available tool to measure fibre tracts in vivo - in planning and performing neurosurgical procedures for brain tumours.
APPROACH
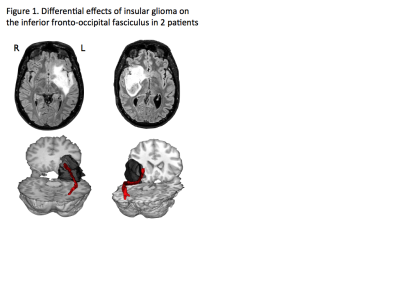
White matter tracts are visually indistinguishable from each other under the surgical microscope. Even small amounts of damage to certain fibres can cause serious disability in stroke (4) and degenerative conditions (5). Accordingly, the presence of behaviourally-important tracts limits neurosurgical removal of a brain tumour more often than the involvement of specific areas of cortex (3). Brain tumours directly affect fibre tracts in various ways. The most common type of tumour arising within the brain, glioma, often encases or infiltrates fibre tracts, or may displace or compress these (6) (Fig 1). The relationship between an individual tumour and neighbouring fibre tracts can therefore determine the likely success of surgery and what, if any, behavioural risks arise.
With the development of MRI techniques sensitive to the orientation of water diffusion in the brain it became possible for the first time to study fibre tracts in the living human brain (7). The ability to visualize the main direction of water diffusion found immediate clinical use to examine deformation of motor fibre tracts by a tumour (8-10). With the development of mathematical models to model fibre trajectories, tractography gained clinical popularity as a tool to pre-operatively predict the behavioural risks of surgery and inform the safest surgical access routes. But tractography is based on mathematical models, applied to an indirect measure of anatomy. How diffusion signals are measured and modeled is still not standardized, and the approach used often reflects what is available or familiar to the user. The use of tractography in neurosurgical settings, therefore, requires careful consideration of methodological choices and pathological confounds that may affect its clinical accuracy and predictive value (11).
1. Spatial accuracy
The importance of choice of tractography model on fibre reconstructions is frequently highlighted. Deterministic algorithms produce visually appealing results that continue into cortex but tend to yield more false negative findings in surgical populations when compared to probabilistic approaches (11).
Among the most important pathological confounds is oedema surrounding a tumour. Oedema alters the diffusion properties upon which tractography is based, and consequently can reduce the accuracy of tractography results by preventing complete reconstruction of tracts or inducing an anatomically implausible route.
Notwithstanding these challenges, tractography shows systematically high reproducibility regardless of the tractography method used for central portions of bundles such as the corticospinal tract (12). Continuous advances in acquisition methods and reconstruction algorithms promise improved differentiation of crossing fibres, detection of cortical fibre terminations (13, 14), and tracking through areas of oedema (15, 16) needed to maximise tractography accuracy.
2. Predictive value: Tractography results should also be clinically meaningful; i.e. correctly predict behavioural risks. A difficult challenge is how to clinically validate tractography (17). The spatial location of a tract does not confirm its function. Therefore, surgery to remove tumours near eloquent brain structures is often performed with the patient awake, so as to monitor and protect essential tissue. Direct electrical stimulation confirms the contribution of fibre tracts to key behaviours (18). Tractography results can be super-imposed onto surgical navigation scans and compared to locations of stimulation-provoked behavioural change. However, the function supported by a tract may be unknown, or may vary along the length of a tract (19). Crucially, removing a tumour mass can result in substantial brain shift (20). Correspondence between the site of behavioural errors in surgery and the location where tracts were located pre-operatively will therefore vary depending on the amount of brain shift and whether techniques are available (e.g. intraoperative MRI (20)) to try to correct for it.
Instead, clinical evaluation of tractography should assess how well it pre-operatively a) predicts functional limitations to tumour removal, b) which functions are at risk, and c) whether it its use reduces postoperative morbidity. Very few randomised trials have attempted to answer these questions. Fewer post-surgical deficits in movement (21), and in vision (22,23), have been reported when neuronavigation included tractography, than without. Randomised studies have not yet been reported for language tracts. However, a recent retrospective analysis reported intact language functions in patients with intact speech tracts, whereas persistent language deficits was predicted by surgical damage to portions of the superior longitudinal fasciculus (24). For semantic language processing, tractography has shown a high predictive value for functional boundaries identified using brain stimulation as well as amount of tumour removal achieved (25).
DISCUSSION
Fifteen years after its inception, tractography is well established as a reliable non-invasive tool to map fibre tracts in individual brains. Direct clinical applications remain limited due to unique considerations of tractography in the presence of pathology. Yet, with careful application, tractography has shown direct use in neurosurgical tumour planning.
By pre-operatively identifying the proximity of fibre tracts, tractography informs discussions with patients about treatment choices in the context of their current performance and future outlook. For some patients, an indication that essential tracts will limit how much tumour that can be removed could allow quicker stratification to alternative (radio/chemo-)therapy. In other cases, when surgery should be performed awake, tractography can focus brain stimulation, and thus reduce seizure risks and patient discomfort associated with extended stimulation (6). Tractography may have future applications in further reducing cognitive and / or neurological morbidity with emerging minimally invasive approaches such as endoscopy and laser interstitial thermal therapy (26). In spite of active application, there remains remarkably little standardisation in the acquisition and reconstruction of diffusion data for surgical indications. Such community standards are sorely needed in order to support large scale studies assessing the real impact of tractography for maximizing and sustaining quality of life at the individual patient level.
Acknowledgements
No acknowledgement found.References
1. Capelle L, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. Journal of neurosurgery. 2013; 118(6): 1157-68.
2. Li YM, et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? Journal of neurosurgery. 2016; 124(4): 977-88.
3. Herbet G, et al. Mapping neuroplastic potential in brain-damaged patients. Brain : a journal of neurology. 2016; 139(Pt 3): 829-44.
4. Fisher CM. Capsular infarcts: the underlying vascular lesions. Archives of neurology. 1979; 36(2): 65-73.
5. Ciccarelli O, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain : a journal of neurology. 2006; 129(Pt 7): 1859-71.
6. Bello L, et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations. Neurosurgical focus. 2010; 28(2): E6.
7. Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature reviews Neuroscience. 2003; 4(6): 469-80.
8. Wieshmann UC, et al. Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumour. Journal of neurology, neurosurgery, and psychiatry. 2000; 68(4): 501-3.
9. Holodny AI, et al. Identification of the corticospinal tracts achieved using blood-oxygen-level-dependent and diffusion functional MR imaging in patients with brain tumors. AJNR American journal of neuroradiology. 2001; 22(1): 83-8.
10. Mori S, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Annals of neurology. 2002; 51(3): 377-80.
11. Bartsch AJ, et al. Presurgical Tractography Applications. In: Johansen-Berg H, Behrens TE, editors. Diffusion MRI: From Quantitative Measurement to In-Vivo Neuroanatomy. 2 ed; 2014.
12. Pujol S, et al. The DTI Challenge: Toward Standardized Evaluation of Diffusion Tensor Imaging Tractography for Neurosurgery. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2015; 25(6): 875-82.
13. Bucci M, et al. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. NeuroImage Clinical. 2013; 3: 361-8.
14. Mandelli ML, et al. Quantifying accuracy and precision of diffusion MR tractography of the corticospinal tract in brain tumors. Journal of neurosurgery. 2014; 121(2): 349-58.
15. Pasternak O, et al. Free water elimination and mapping from diffusion MRI. Magnetic resonance in medicine. 2009; 62(3): 717-30.
16. Kuhnt D, et al. Fiber tractography based on diffusion tensor imaging compared with high-angular-resolution diffusion imaging with compressed sensing: initial experience. Neurosurgery. 2013; 72 Suppl 1: 165-75.
17. Voets NL, et al. Brain white matter fibre tracts: a review of functional neuro-oncological relevance. Journal of neurology, neurosurgery, and psychiatry. 2017.
18. Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nature reviews Neurology. 2015; 11(5): 255-65.
19. Mandonnet E, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain : a journal of neurology. 2007; 130(Pt 3): 623-9.
20. Nimsky C, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005; 56(1): 130-7; discussion 8.
21. Wu JS, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery. 2007; 61(5): 935-48; discussion 48-9.
22. Coenen VA, et al. Diffusion-weighted imaging-guided resection of intracerebral lesions involving the optic radiation. Neurosurgical review. 2005; 28(3): 188-95.
23. Winston GP, et al. Preventing visual field deficits from neurosurgery. Neurology. 2014; 83(7): 604-11.
24. Caverzasi E, et al. Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas. Journal of neurosurgery. 2015: 1-13.
25. Martino J, et al. Subcortical anatomy as an anatomical and functional landmark in insulo-opercular gliomas: implications for surgical approach to the insular region. Journal of neurosurgery. 2015; 123(4): 1081-92.
26. Sloan AE, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. Journal of neurosurgery. 2013; 118(6): 1202-19.