The Payoff for the Pain
1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Erwin L. Hahn Institute for MRI, University of Duisburg-Essen, Essen, Germany
Synopsis
This presentation will emphasize some clinical and scientific applications in human ultra-high field MR that particularly benefit from the changing physical characteristics at high magnetic fields, including susceptibility-weighted imaging and phase contrast techniques, imaging with X-nuclei, MR spectroscopy, and CEST imaging.
Magnetic resonance imaging and spectroscopic techniques are widely used in humans for both clinical diagnostic applications as well as in basic research areas such as cognitive neuroimaging. In recent years, new human MR systems have become available operating at static magnetic fields of 7 Tesla or higher (≥ 300 MHz proton frequency). Imaging human-sized objects at such high frequencies presents several challenges including non-uniform radiofrequency fields, more severe susceptibility artifacts, and higher radiofrequency energy deposition in the tissue. On the positive side are gains in signal-to-noise or contrast-to-noise ratio that allow finer structures to be visualized and smaller physiological effects to be detected.
The advantages and difficulties for MR when moving to higher magnetic fields have been summarized in several review articles [1-4], and Table 1 summarizes a variety of physical characteristics that affect MR imaging and MR spectroscopy (MRS) at high magnetic fields. In a few cases the changes in these parameters are decisively positive. In a few other cases the changes are decisively negative. However, for a majority of the changes the impact depends on the goal and method of the underlying imaging experiment: in some cases the effect is beneficial, in others a hindrance. As almost any given experiment is affected by a complex interplay between multiple parameters, it is not possible to directly translate approaches from lower fields strengths without adjusting and optimizing imaging parameters and where necessary introducing new imaging hardware to achieve the full potential at UHF.
This presentation will emphasize some clinical and scientific applications in human ultra-high field MR that particularly benefit from the changing physical characteristics at high magnetic fields, including susceptibility-weighted imaging (Fig. 1) and phase contrast techniques, imaging with X-nuclei (Fig. 2), MR spectroscopy, and CEST imaging. It will be discussed how methodological developments such as parallel transmission and motion correction can be leveraged to explore the full potential of higher magnetic fields.
The versatility and power of the MR technique will continue to drive the quest for ever higher magnetic fields to help unravel important unanswered questions about healthy physiology, pathological processes, brain function, and ageing. The increase in SNR and CNR at UHF is a universal benefit that can not only improve existing applications including high-resolution structural imaging and susceptibility-weighted imaging, but also facilitate completely new application domains for MR, particularly in spectroscopy and CEST imaging with 1H as well as imaging and spectroscopy with X-nuclei. With the availability of UHF, these applications may even be able to make the leap from research to clinical diagnostic use.
Acknowledgements
No acknowledgement found.References
[1] O. Kraff, A. Fischer, A.M. Nagel, C. Monninghoff, M.E. Ladd, MRI at 7 Tesla and above: demonstrated and potential capabilities, J Magn Reson Imaging, 41 (2015) 13-33.
[2] O. Kraff, H.H. Quick, 7T: Physics, safety, and potential clinical applications, J Magn Reson Imaging, 46 (2017) 1573-1589.
[3] E. Moser, F. Stahlberg, M.E. Ladd, S. Trattnig, 7-T MR--from research to clinical applications?, NMR Biomed, 25 (2012) 695-716.
[4] K. Ugurbil, Imaging at ultrahigh magnetic fields: History, challenges, and solutions, Neuroimage, 168 (2018) 7-32.
[5] J.M. Theysohn, O. Kraff, S. Maderwald, M. Barth, S.C. Ladd, M. Forsting, M.E. Ladd, E.R. Gizewski, 7 Tesla MRI of microbleeds and white matter lesions as seen in vascular dementia, Journal of Magnetic Resonance Imaging, 33 (2011) 782-791.
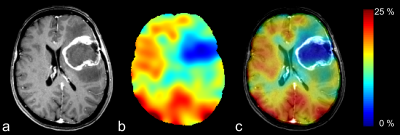
[6] S.H. Hoffmann, A. Radbruch, M. Bock, W. Semmler, A.M. Nagel, Direct O-17 MRI with partial volume correction: first experiences in a glioblastoma patient, Magnetic Resonance Materials in Physics Biology and Medicine, 27 (2014) 579-587.
Figures