Upper-Extremity Peripheral Nerve Imaging with US: European Perspective
1University of Genova, Italy
Synopsis
The value of US to assess the most common disorders affecting peripheral nerves in the upper extremity, including compressive neuropathies, polyneuropathies, traumatic injuries, tumours and tumour-like lesions, will be discussed. A careful US approach with thorough understanding of soft-tissue planes and extensive familiarity with anatomy are prerequisites for obtaining reliable information regarding such kind of examination. US can provide a low-cost and noninvasive imaging, speed of performance, and important advantages over MR imaging, including higher spatial resolution and ability to explore long segments of nerve trunks in a single study and to examine tissues in both static and dynamic states with real time scanning.
Introduction
There is expanding evidence base to support the use of ultrasound (US) as an imaging tool to evalutare the peripheral nervous system. However, the highly operator-dependent nature and level of technical expertise required to perform an adequate US assessment of peripheral neuropathies means that appropriate training and an in-depth knowledge of anatomy and clinical findings are required. The value of US to assess the most common disorders affecting peripheral nerves in the upper extremity, including compressive neuropathies, polyneuropathies, nerve injuries, tumours and tumour-like lesions, will be discussed. A careful US approach with thorough understanding of soft-tissue planes and extensive familiarity with anatomy are prerequisites for obtaining reliable information regarding such kind of examination. With these credentials, US performed by a skilled operator provides a low-cost and noninvasive imaging, speed of performance, and important advantages over MR imaging, including higher spatial resolution and ability to explore long segments of nerve trunks in a single study and to examine tissues in both static and dynamic states with real time scanning.Compressive Neuropathies
Based on US assessment, nerve compressive syndromes can be divided in three classes:
- class-1 - large nerves (e.g. median, ulnar). US evaluation requires mid-range equipments and probe frequency range up to 12-13MHz. Diagnosis is based on pattern recognition analysis and - for the carpal and cubital tunnels - on calculation of the nerve cross-sectional area (CSA);
- class-2 - small nerves (e.g. posterior and anterior interosseous, musculocutaneous, distal divisional branches of large nerves). High-end equipments and probe frequency range up to 17-20MHz are required. Diagnosis is based on pattern recognition analysis and calculation of the nerve diameter because CSA measurements cannot be applied (too small nerve size).
- class-3 - nerves that are poorly visible or non-detectable with US due to a too deep course or intervening bone (e.g. segments of the axillary nerve). For motor/mixed nerves, the US diagnosis is based on indirect evaluation of muscles. US detection of denervation is based on loss in bulk and hyperechoic appearance of the affected muscles. US is not as accurate as MR imaging to differentiate the process of early denervation related to accumulation of intramuscular extracellular edema from fatty atrophy.
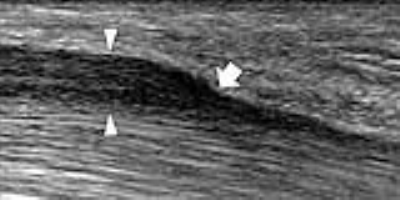
Regardless the entrapment site, the US signs of compressive neuropathy are stereotypical. They include: nerve flattening at the compression point and nerve swelling proximal or (less commonly) distal to it. The transition between swollen and flattened segments is abrupt and referred to as the “notch sign” (Fig. 1). For quantification of findings, the most relevant measure is the nerve CSA (for class-1 nerves) or the maximum nerve diameter (for class-2 nerves). The CSA should be sampled at the site where the nerve is maximally enlarged and histopathologic changes are most severe. In the early phases of compression, intraneural edema and venous congestion are the main factors leading to the nerve enlargement. A positive correlation exists between nerve CSA and severity of EMG findings. In severe/longstanding compressions, the nerve echotexture may appear massively hypoechoic with loss of the fascicular pattern due to swelling of the fascicles and reduced echogenicity of the epineurium. Irreversible intraneural fibrosis may occur. Different from early disease, nerves with fibrotic changes remain swollen after decompressive surgery and get poor functional improvement.
Polyneuropathies
Inherited Disorders
Charcot-Marie-Tooth disease includes demyelinating (CMT-1) and axonal (CMT-2) forms. Marked generalized fascicular enlargement reflecting "onion bulb" Schwann cell hypertrophy due to attempted remyelination typifies CMT-1A. Axonal forms (CMT-2) are more heterogeneous and show mild nerve enlargement. Hereditary Neuropathy with Liability to Pressure Palsies (HNPP) is a congenital disorder characterized by segmental demyelination and tomaculous “sausage-shaped” myelin sheath swelling. US shows multifocal nerve enlargement (tomacula) following trivial trauma.
Immune-Mediated (dysimmune) Polyneuropathies
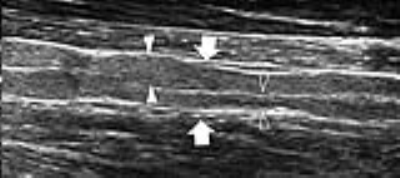
In Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) US reveals segmental nerve abnormalities where conduction blocks are identified at electrophysiology. Fascicles may alternate thickened and thinned segments (Fig. 2). In Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP or Guillain-Barré syndrome), US is able to detect focal changes in nerve/fascicle thickness in early disease when electrophysiology is still negative. US shows reduced fascicular swelling during treatment before neurophysiological improvement appears. In multifocal motor neuropathy (MMN), US can detect multifocal nerve swellings.
Leprosy (Hansen disease)
Leprosy is a multifaceted infectious disease caused by Mycobacterium leprae in which skin and nerves in the extremities are specifically involved. It represents the most diffuse neuropathy worldwide. US can reveal markedly swollen nerves with loss of the fascicular echotexture and thickened epineurium. Acute neuritic phases may be predicted by intense intraneural hyperemia at Doppler imaging, the so called “nerve inferno”, a sign suggesting rapid progression of nerve damage and the need for immediate immunosuppressive therapy with corticosteroids.
Nerve Injuries
Penetrating trauma, stretching and contusion are the pathomechanisms of nerve injuries.
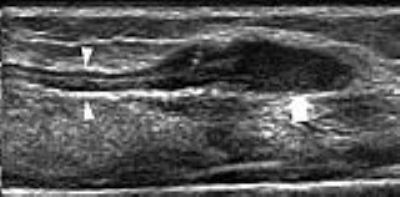
Penetrating Injuries. In complete nerve transection, stump (terminal) neuromas develop as oval hypoechoic masses in continuity with the edges of the severed nerve. Detection of terminal neuromas may help to map the location of the nerve ends, which may be displaced and retracted away from the site of injury (Fig. 3). Surgical repair after nerve injury involves excision of the neuroma; the gap length, therefore, should include the end-to-end distance plus the neuroma length. In partial nerve tears, US can estimate the percentage of injured and preserved fascicles. A spindle neuroma may develop in continuity with the injured nerve. US is unable to assess the status of the fascicles within the neuroma and cannot predict outcome and recovery time.
Stretching Injuries. In significant trauma without laceration, a fusiform hypoechoic swelling with loss of the fascicular echotexture can develop reflecting a spindle neuroma related to intraneural fibrosis to a non-disrupted nerve trunk. An oblique course and the presence of fixation points make nerves vulnerable to stretching injuries. These lesions also occur where nerves pierce fascial planes.
Contusion Trauma. Contusion trauma most often occurs where nerves run closely apposed to bony surfaces (e.g. superficial bony prominences, spurs, osteophytes) and are vulnerable to external pressure. Contusion leads to segmental nerve thickening with swollen fascicles and thickened epineurium. Similar findings occur in ulnar nerve instability at the elbow (friction neuritis).
Nerve Tumors and Tumor-like Lesions
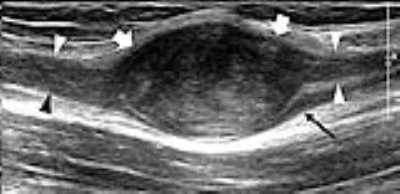
Neurogenic Histotypes. Benign peripheral nerve sheath tumors include two main benign forms: schwannoma (also known as neurinoma or neurilemmoma) and neurofibroma. The US diagnosis basically relies on detection of a soft-tissue mass in continuity with a nerve at its proximal and distal poles. This feature may not be seen in cases of tumors arising from too small and distal nerve branches. A rim of fat, the “fat-split sign”, suggesting an origin of the mass in the intermuscular space about the neurovascular bundle is another feature of value. Some differential features have been reported between schwannomas and neurofibromas. Schwannomas are eccentric ovoid masses, whereas neurofibromas encase the fascicles of the parent nerve developing in a fusiform shape (Fig. 4). Neurofibromas may show a “target sign” consisting of a hyperechoic (fibrous) core and a hypoechoic (myxomatous tissue) rim. Plexiform neurofibromas are pathognomonic for type-1 neurofibromatosis (NF-1), usually involving a long nerve segment and its branches with tortuous expansion, and their gross appearance has been described as a "bag of worms". Malignant forms tend to be larger (>5cm) than benign hystotypes and often appear as inhomogeneous hypoechoic masses with calcifications, areas of internal bleeding and necrosis and indistinct margins as a result of their infiltrative growth. Despite these differences, a clear separation between benign and malignant forms is frequently unfeasible with US.
Non-Neurogenic Intraneural Masses. Non-neural sheath nerve masses (e.g. lipomas, paragangliomas, hemangiomas, lymphomas, extrinsic neoplasms and ganglion cysts) may either originate or infiltrate or adhere to a nerve. Fibrolipomatous hamartoma is a developmental tumor-like nerve disorder related to accumulation of mature fat and fibroblasts in the epineurium that often presents at birth or during early childhood. The US appearance of fibrolipomatous hamartoma reflects its histopathology with increased epineurial fat surrounding slightly enlarged fascicles. Intraneural ganglia appear as elongated cystic masses contained within the nerve sheath. The pathogenesis is related to penetration of joint fluid into the nerve trunk through a small articular (capsular) branch. The fluid is pumped within the articular branch through small capsular fenestrations and, after exiting the joint, it progresses upstream to reach the main nerve trunk. Intraneural ganglia do not have a fibrous/synovial lining.
Acknowledgements
No acknowledgement found.References
- Silvestri E, Martinoli C, Derchi LE et al. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995; 197:291-296.
- Kermarrec E, Demondion X, Khalil et al. Ultrasound and magnetic resonance imaging of the peripheral nerves: current techniques, promising directions, and open issues. Semin Musculoskelet Radiol 2010; 14:463-472.
- Martinoli C, Bianchi S, Gandolfo N et al. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. RadioGraphics 2000; 20:199-217.
- Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol 1999; 173:681-684.
- Martinoli C, Bianchi S, Pugliese F et al. Sonography of entrapment neuropathies in the upper limb (wrist excluded). J Clin Ultrasound 2004; 32:438-450.
- Hobson-Webb LD, Padua L, Martinoli C. Ultrasonography in the diagnosis of peripheral nerve disease. Expert Opin Med Diagn 2012; 6:457-471.
- Zaidman CM, Al-Lozi M, and Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve 2009; 40:960-966.
- Chiou HJ, Chou YH, Chiou SY, et al. Peripheral nerve lesions: role of high-resolution US. RadioGraphics 2003; 23:E15.
- Abreu E, Aubert S, Wavreille G et al. Peripheral nerve tumors and tumor-like neurogenic lesions. Eur J Radiology 2013; 82:38-50.
Figures