Alcohol: WM & Alcohol
1Stanford University School of Medicine, United States, 2SRI International, Menlo Park, CA, United States
Synopsis
Brain imaging technology has allowed researchers to conduct rigorous studies of the dynamic course of alcoholism through periods of drinking, sobriety, and relapse and to gain insights into the effects of chronic alcoholism on the human brain. Magnetic resonance imaging (MRI) studies have distinguished alcohol-related brain effects that are permanent from those that are reversible with abstinence. In support of postmortem neuropathological studies showing degeneration of white matter, MRI studies have shown a specific vulnerability of white matter to chronic alcohol exposure. Such studies have demonstrated white-matter volume deficits as well as damage to selective gray-matter structures. Diffusion tensor imaging (DTI), by permitting microstructural characterization of white matter, has extended MRI findings in alcoholics. This lecture will focus on DTI findings in neurological disorders that commonly co-occur with alcoholism, including Wernicke's encephalopathy, Korsakoff's syndrome, and hepatic encephalopathy. Also reviewed are neuroimaging findings in animal models of alcoholism and related neurological disorders. The dynamic course of alcoholism presents a unique opportunity to examine brain structural and functional repair and recovery.
Syllabus
In 2012, 3.3 million (6%) worldwide deaths were attributable
to alcohol consumption. Globally, alcohol misuse is the fifth leading risk
factor for premature death and disability; among those aged 15 - 49 years, it
is the first (1). It is
therefore critical for clinicians to be attentive of the scope of the problem
and consider the extent of alcohol use in all patients. Since heavy alcohol use
has been shown to affect brain imaging metrics (including brain volume,
MRS-detectable metabolites, and diffusion tensor imaging measures), awareness
of the effects of alcohol on the brain is also critical for all personnel
involved with brain MR imaging.
Those who attend this lecture will get an overview of
alcohol-related diseases that have clear neuroimaging signature. They will also
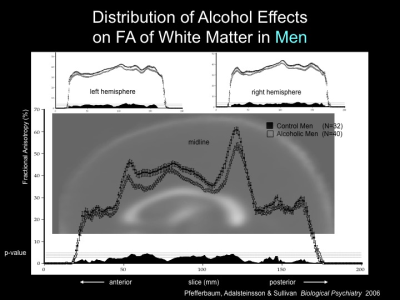
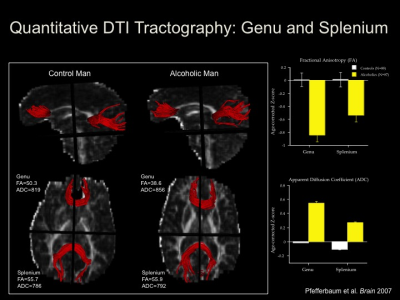
gain a better understanding of the permanent and transient effects of alcohol
misuse on brain structural integrity. This information will also apprise
researchers of the importance of interrogating volunteers about drinking
consumption recency, frequency, and amount before assuming that a volunteer is
truly an "unaffected healthy participant."
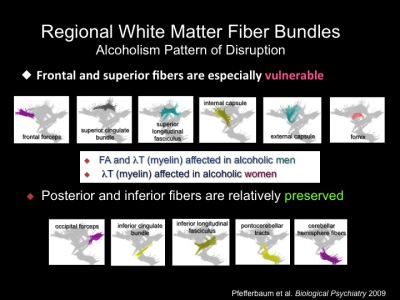
In support of postmortem neuropathological studies
showing degeneration of white matter, neuroimaging studies have shown a
specific vulnerability of regional white matter macrostructure using
conventional MRI and microstructure using diffusion tensor imaging (DTI) to
chronic alcohol exposure. Such studies have demonstrated white-matter volume
deficits that typically affect anterior brain systems. Gray matter tissue is
also affected in chronic alcoholism where frontal, parietal, and cerebellar
systems are commonly affected. Unlike many disorders affecting brain structure,
prolonged sobriety can result in gray matter volume expansion and improvement
in microstructural measures of white matter fiber tract integrity. Because of
the potential for at least partial reversal of tissue volume deficits, it is
preferable to characterize deficits as shrinkage rather than loss, which
denotes a permanent condition.
In order
to evaluate its central nervous system (CNS) effects, researchers distinguish
“uncomplicated alcoholism” from the various clinically diagnosable consequences
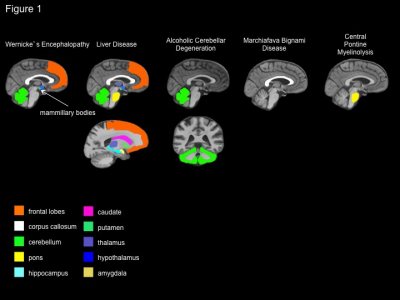
of chronic alcohol consumption, including Wernicke’s encephalopathy (WE),
Korsakoff’s syndrome (KS), [Wernicke-Korsakoff syndrome (WKS) is used to refer
to the presence of both WE and KS because WE is a common antecedent to KS], hepatic
encephalopathy (HE), central pontine myelinolysis (CPM), and Marchiafava–Bignami
disease (MBD). The evaluation of clinically defined syndromes associated with
chronic alcoholism, each with a relatively unique radiologic signature,
provides guideposts for the interrogation of the brain in uncomplicated
alcoholism.
Human studies offer a full depiction of
the consequences of chronic alcohol exposure but are limited by ethical
considerations. That is, rigorous experimentation requires the ability to
control for relevant variables such as the premorbid condition of the brain.
The wide variation (or heterogeneity) of alcoholic populations examined with
respect to genetic predisposition, age of onset, pattern of drinking, frequency
of withdrawals, length of sobriety, nutritional, and hepatic status has
hampered systematic attempts to isolate specific brain regions and mechanisms
affected by alcohol, per se. This heterogeneity, and the complexity that it
introduces, makes characterizing the disorder a research challenge. Animal
models, in contrast to the indefinite natural course of alcohol use in humans,
allow researchers to determine alcohol toxicity by controlling multiple
genetic, environmental, sex, and alcohol consumption factors. Animal models
permit the study of underlying mechanisms, thereby enhancing interpretations of
findings in human studies
DTI results from alcoholics with WKS (2, 3), HE (4-8), CPM (9, 10), and MBD (11-16) will be
reviewed. DTI has revealed microstructural damage related to alcoholism in CNS
areas that appear intact in structural MRI analyses (17-19). These results
will be discussed.
DTI data have been collected in animal
models of WE (20-22). In one study,
animals with WE induced by thiamine deficiency were imaged at baseline,
presymptomatic stage (day 10), symptomatic stage (days 12 and 14), and after
recovery on days 31 and 87. A decrease in fractional anisotropy (FA) in the
inferior colliculi was first noted on day 10 but showed recovery on day 87. On
the other hand, the FA decrease in the thalamus first noted on day 12 persisted
through day 87.
Affects of various ethanol (EtOH) exposure
protocols on rat brain DTI metrics will be covered (23-25). For example,
adult rats exposed to a single dose of EtOH showed a slight and transient
reduction, relative to unexposed rats, in apparent diffusion coefficient in
brainstem (23), frontal lobe, hippocampus, thalamus,
and cerebellum (24). Binge EtOH
followed by a week of sobriety resulted in rapidly reversible decreases in FA
in callosal genu and fimbria-fornix but not splenium; and increases in mean
diffusivity selective to the fimbria-fornix (25).
Acknowledgements
No acknowledgement found.References
1. World Health Organization. Global Status Report on Alcohol and Health. 2014. 2. Segobin S, Ritz L, Lannuzel C, Boudehent C, Vabret F, Eustache F, et al. Integrity of white matter microstructure in alcoholics with and without Korsakoff's syndrome. Human brain mapping. 2015;36(7):2795-808. 3. Nahum L, Pignat JM, Bouzerda-Wahlen A, Gabriel D, Liverani MC, Lazeyras F, et al. Neural Correlate of Anterograde Amnesia in Wernicke-Korsakoff Syndrome. Brain topography. 2015;28(5):760-70. 4. Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, et al. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology (Baltimore, Md. 2006;43(4):698-706. 5. Kumar R, Gupta RK, Elderkin-Thompson V, Huda A, Sayre J, Kirsch C, et al. Voxel-based diffusion tensor magnetic resonance imaging evaluation of low-grade hepatic encephalopathy. J Magn Reson Imaging. 2008;27(5):1061-8. 6. Miese F, Kircheis G, Wittsack HJ, Wenserski F, Hemker J, Modder U, et al. 1H-MR spectroscopy, magnetization transfer, and diffusion-weighted imaging in alcoholic and nonalcoholic patients with cirrhosis with hepatic encephalopathy. Ajnr. 2006;27(5):1019-26. 7. Ahluwalia V, Wade JB, Moeller FG, White MB, Unser AB, Gavis EA, et al. The etiology of cirrhosis is a strong determinant of brain reserve: A multimodal magnetic resonance imaging study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015;21(9):1123-32. 8. Mardini H, Smith FE, Record CO, Blamire AM. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. Journal of hepatology. 2011;54(6):1154-60. 9. Min Y, Park SH, Hwang SB. Corticospinal tract and pontocerebellar fiber of central pontine myelinolysis. Ann Rehabil Med. 2012;36(6):887-92. 10. Nair SR, Ramli NM, Rahmat K, Mei-Ling ST. Central pontine and extrapontine myelinolysis: Diffusion weighted imaging and diffusion tensor imaging on follow-up. Neurology India. 2012;60(4):426-8. 11. Menegon P, Sibon I, Pachai C, Orgogozo JM, Dousset V. Marchiafava-Bignami disease: diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology. 2005;65(3):475-7. 12. Tuntiyatorn L, Laothamatas J. Acute Marchiafava-Bignami disease with callosal, cortical, and white matter involvement. Emerg Radiol. 2008;15(2):137-40. 13. Bano S, Mehra S, Yadav SN, Chaudhary V. Marchiafava-Bignami disease: Role of neuroimaging in the diagnosis and management of acute disease. Neurology India. 2009;57(5):649-52. 14. Wenz H, Eisele P, Artemis D, Forster A, Brockmann MA. Acute Marchiafava-Bignami disease with extensive diffusion restriction and early recovery: case report and review of the literature. J Neuroimaging. 2014;24(4):421-4. 15. Pacheco FT, Rego MM, do Rego JI, da Rocha AJ. "Ears of the Lynx" Sign in a Marchiafava-Bignami patient: Structural Basis and Fiber-Tracking DTI Contribution to the Understanding of this Imaging Abnormality. J Neuroimaging. 2012;24(2):205-7. 16. Sair HI, Mohamed FB, Patel S, Kanamalla US, Hershey B, Hakma Z, et al. Diffusion tensor imaging and fiber-tracking in Marchiafava-Bignami disease. J Neuroimaging. 2006;16(3):281-5. 17. Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of aging. 2006;27(7):994-1009. 18. Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological psychiatry. 2006;59(4):364-72. 19. Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, et al. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism, clinical and experimental research. 2003;27(2):301-9. 20. Dror V, Eliash S, Rehavi M, Assaf Y, Biton IE, Fattal-Valevski A. Neurodegeneration in thiamine deficient rats-A longitudinal MRI study. Brain research. 2010;1308:176-84. 21. Dror V, Rehavi M, Biton IE, Eliash S. Rasagiline prevents neurodegeneration in thiamine deficient rats-a longitudinal MRI study. Brain research. 2014;1557:43-54. 22. Eliash S, Dror V, Cohen S, Rehavi M. Neuroprotection by rasagiline in thiamine deficient rats. Brain research. 2009;1256:138-48. 23. Kong L, Lian G, Zheng W, Liu H, Zhang H, Chen R. Effect of alcohol on diffuse axonal injury in rat brainstem: diffusion tensor imaging and aquaporin-4 expression study. Biomed Res Int. 2013;2013:798261. 24. Liu H, Zheng W, Yan G, Liu B, Kong L, Ding Y, et al. Acute ethanol-induced changes in edema and metabolite concentrations in rat brain. Biomed Res Int. 2014;2014:351903. 25. Pfefferbaum A, Zahr NM, Mayer D, Rohlfing T, Sullivan EV. Dynamic responses of selective brain white matter fiber tracts to binge alcohol and recovery in the rat. PLoS One. 2015;10(4):e0124885.Figures