5660
The role of driver mutations in pediatric diffuse gliomas with histone H3 K27M mutation1University of California San Francisco, San Francisco, CA, United States
Synopsis
In this educational exhibit we will describe the imaging features of the new diagnostic entity based on 2016 CNS WHO classification – diffuse midline glioma with histone H3 K27M mutation. We will review the genetics of these tumors and will describe the common driver mutations that are detected. We will contrast the molecular signature of these tumors to hemispheric gliomas that have different imaging features and different outcomes.
Teaching points
1. To review the 2016 WHO classification of gliomas, with addition of a new category “diffuse midline glioma, H3 K27M mutant “
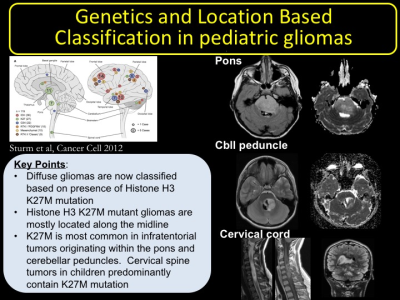
2. To highlight the characteristics of histone H3 K27M mutant diffuse gliomas based on their location. Infratentorial gliomas centered within pons and cerebellar peduncle, cervical spine gliomas, and supratentorial gliomas centered within the thalamus will be highlighted.
3. To highlight the imaging characteristics of histone H3 wildtype gliomas that are predominantly located within the cerebral hemispheres and to describe the genetic mutations involved in hemispheric pediatric gliomas
4. To describe the recent literature updates on the driver mutations found in pediatric diffuse gliomas including ACVR1, PPM1D, PIK3CA, and SETD2.
Table of Contents/Outline
I. Overview of the genetic differences between the pediatric diffuse gliomas, adult glioblastomas, and adult secondary glioblastomas with respect to presence of mutations in EGFR, p53, Histone H3, IDH1, and 1p19q deletion.
II. Review of the 2016 WHO classification of diffuse gliomas, with inclusion of “diffuse midline glioma, H3 K27M mutant”.
III. Review of the molecular biology of histone families and the role mutations in histone H3 plays a role in tumorigenesis.
IV. Review of classification of diffuse gliomas based on their location within the brain. Histone H3 K27M mutant and wildtype gliomas will be compared at different locations including pons, cerebellar peduncle, and thalamus. Histone H3 wildtype gliomas centered within cerebellar hemispheres will be presented to contrast the midline gliomas.
V. Review and compare the types of mutations involved in diffuse midline gliomas and hemispheric gliomas
Acknowledgements
National Institutes of Health Biomedical Imaging and Bioengineering T32 grant (Grant Number 5T32EB001631-12)References
1. Aboian MS, Solomon DA, Felton E, et al. Imaging Characteristics of Pediatric Diffuse Midline Gliomas with Histone H3 K27M Mutation. AJNR American journal of neuroradiology 2017; 38(4): 795-800. 2. Banan R, Hartmann C. The new WHO 2016 classification of brain tumors-what neurosurgeons need to know. Acta neurochirurgica 2017; 159(3): 403-18.
3. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta neuropathologica 2015; 130(6): 815-27.
4. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012; 482(7384): 226-31.
5. Solomon DA, Wood MD, Tihan T, et al. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol 2015.
6. Kline CN, Joseph NM, Grenert JP, et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro-oncology 2017; 19(5): 699-709.
7. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012; 22(4): 425-37.