5645
Spectral Model Dependent Quantification of Triglyceride Composition using Chemical Shift Encoded Magnetic Resonance Imaging1Physics and Astronomy, University of Georgia, Athens, GA, United States, 2Bio-Imaging Research Center, University of Georgia, Athens, GA, United States, 3Biochemistry and Molecular Biology, University of Georgia, Athens, GA, United States, 4Center for Molecular Medicine, University of Georgia, Athens, GA, United States
Synopsis
Dynamic processes such as brown adipose tissue (BAT) activation and white adipose tissue (WAT) beiging have been shown to change triglyceride composition. Therefore, accurate spatial quantification of triglyceride composition is important for the monitoring of these processes. Presented here is an evaluation of the performance of various fat spectral models on the quantification of triglyceride composition using chemical shift encoded magnetic resonance imaging (CSE-MRI). Variations as large as 45% and less than 2.82% are observed in the average estimations of triglyceride composition and proton density fat fraction (PDFF), respectively. Estimations obtained using a material specific model correlate better with spectroscopy estimations than other examined models.
Purpose
To explore, at a high field strength of 7T, the performance of various fat spectral models on the quantification of triglyceride composition and proton density fat fraction (PDFF) using chemical-shift encoded MRI (CSE-MRI).Methods
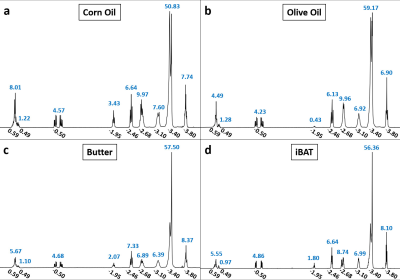
Using a 7T Varian Magnex MRI Scanner, experiments were conducted using a 2D multi-echo gradient-echo sequence. Phantom experiments included three vials containing corn oil, olive oil, and butter placed inside a cylindrical tube filled with agar gelatin. In vivo experiments were performed on the intercapsular brown adipose tissue (iBAT) and inguinal white adipose tissue (igWAT) of 2-month-old C57/BL6 mice (n=2).
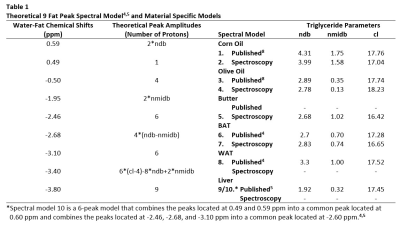
Triglyceride composition and PDFF of each sample was estimated from acquired MR data using various a priori fat spectral models listed in Table 1. The a priori models were used in both the field map and $$$R_{2}^{*}$$$ estimation methods. The field map was estimated using a graphcut algorithm,1,2 and its contribution was removed from the signals prior to $$$R_{2}^{*}$$$ estimation. $$$R_{2}^{*}$$$ was then solved on a voxel by voxel basis using variable projection to perform complex non-linear least squares fitting.1,2 Finally, estimates of triglyceride composition (ndb, nmidb, and cl from Table 1) and PDFF were determined from the total MR signals on a voxel by voxel basis using the previously estimated $$$R_{2}^{*}$$$ and field maps and the mldivide algorithm provided by Matlab (Mathworks Inc.) to solve the linear system of equations described by Peterson et al.,3 which ties information regarding the triglyceride composition to the MR signals using the locations and theoretical amplitudes of the fat resonance peaks.
Proton (1H) NMR spectroscopy experiments were conducted on all three phantom materials using a 300 MHz Varian Mercury Spectrometer and on surgically removed and prepared iBAT tissues from separate C57/BL6 mice of the same age (n=3) using a 600 MHz Varian Inova Spectrometer. Assuming the 9-peak model in Table 1,4,5 the relative amplitudes of the fat peaks for all acquired spectra were determined by performing multiplet analysis using MNOVA (Mestrelab Research), and from these measurements, estimates of ndb, nmidb, and cl were obtained by solving the linear system of equations associated with the theoretical peak amplitudes given in Table 1.4,5 For the iBAT tissues (n=3), the average relative amplitudes were used in estimating ndb, nmidb, and cl.
The variability in the CSE-MRI estimates across spectral models was evaluated by dividing the ranges of the average estimates by their corresponding medians. To determine which spectral models correlate better with spectroscopy estimations, the relative errors of the average CSE-MRI estimations with respect to the spectroscopy estimations were summed across ndb, nmidb, and cl for each model and then scaled between zero and one for each material.
Results
Estimations of triglyceride composition obtained from spectroscopy are given in Table 1 (models 2, 4, 5, and 7), and Figure 1 displays representative spectrums with the chemical shifts and measured relative amplitudes of the 9 fat resonance peaks.
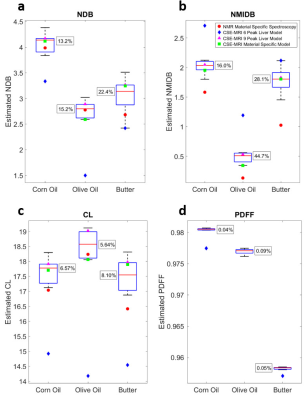
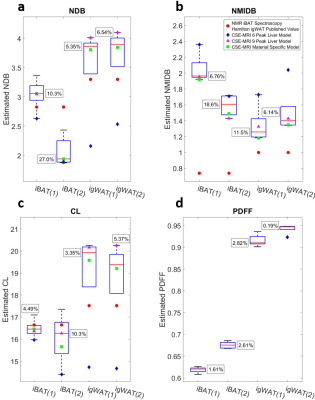
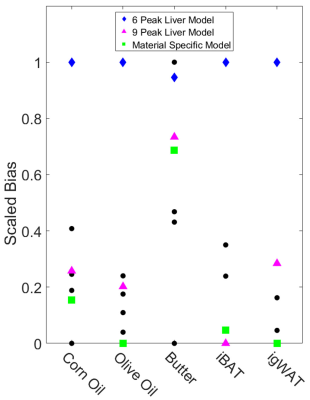
For phantom experiments, models 1-5 and 9-10 were used to estimate triglyceride composition, while models 6-10 were used for the in vivo experiments. Large variations in the estimations of triglyceride composition are observed across spectral models (Figs. 2a-c and 3a-c); however, the variations in the estimations of PDFF are relatively small (Figs. 2d and 3d). The interquartile ranges of the triglyceride composition estimates tend to be greater than the estimations obtained from spectroscopy, and in most cases, the 6-peak model produces estimations that are considered outliers from the rest of the results. When comparing across all three triglyceride composition parameters, use of the material specific spectroscopy model produces either the best or second best correlation with spectroscopy for four out of five of the materials studied in this work (Fig. 4), while the 6-peak liver model produces the worst or second worst correlation for all five materials.
Discussion and Conclusions
At high field strengths, accurate (e.g. 9-peak) material specific fat spectral modeling is necessary to quantify triglyceride composition when using CSE-MRI estimation methods that assume the spectral model to be known as a priori information. This makes the estimation process seem circular; however, opposed to spectroscopy methods, which provide average triglyceride composition estimations, CSE-MRI methods allow for the spatial quantification of triglyceride composition. Alternatively, iterative techniques that do not rely on an a priori spectral model to solve for triglyceride composition may be more effective. The quantification of PDFF, however, is shown to be independent of the chosen a priori spectral model at a high field strength of 7T, which agrees with previously reported results obtained at lower field strengths (e.g. 1.5T and 3T).6,7Acknowledgements
The authors would like to thank Dr. Diego Hernando and Dr. Houchun H. Hu for their willingness to discuss the methods provided in the ISMRM fat-water toolbox (https://www.ismrm.org/workshops/FatWater12/data.htm). We acknowledge the use of this toolbox for some of the estimation methods performed in this work. The project described was supported by Award S10RR023706 from the National Center for Research Resources, the National Institutes of Health.References
1. Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang ZP. Joint estimation of water/fat images and field inhomogeneity map. Magn Reson Med 2008;59(3):571-80.
2. Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med 2010;63(1):79-90.
3. Peterson P, Mansson S. Simultaneous quantification of fat content and fatty acid composition using MR imaging. Magn Reson Med 2013;69(3):688-97.
4. Hamilton G, Smith DL, Jr., Bydder M, Nayak KS, Hu HH. MR properties of brown and white adipose tissues. J Magn Reson Imaging 2011;34(2):468-73.
5. Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed 2011;24(7):784-90.
6. Hong C, Mamidipalli A, Hooker JC, Hamilton G, Wolfson T, Dehkordy SF, Middleton MS, Reeder SB, Loomba R, Sirlin CB. Hepatic MRI-PDFF is positively correlated with R2* across a range of fat spectral models. In Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, USA 2017.
7. Wang X, Hernando D, Reeder SB. Sensitivity of chemical shift-encoded fat quantification to calibration of fat MR spectrum. Magn Reson Med 2016;75(2):845-51.
8. Bydder M, Girard O, Hamilton G. Mapping the double bonds in triglycerides. Magn Reson Imaging 2011;29(8):1041-6.
Figures