5628
3D multi-parameter mapping of the breast with Dixon fat-water separation1Department of Imaging Physics, The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States, 2MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States, 3MR Applications and Workflow, GE Healthcare, Waukesha, WI, United States, 4Department of Radiology, The University of Texas M.D. Anderson Cancer Center, Houston, TX, United States, 5SyntheticMR, Linkoping, Sweden
Synopsis
3D QALAS is a promising new technique that simultaneously maps T1, T2, and PD in a single 3D acquisition. However, the presence of fat can confound multi-parameter mapping of voxels with mixed species. We modified the sequence to acquire dualecho readouts and apply a joint field map estimation across all echoes to produce water-only images for multi-parameter fitting. The technique was applied in the breast and parameter maps were compared with those generated from a 2D unsuppressed acquisition. By eliminating partial volume effects of fat, the technique potentially extends the use of 3D multi-parameter mapping into body, breast, and spine.
Introduction
3D QALAS [1] (3D-quantification using an interleaved Look-Locker acquisition sequence with T2 preparation pulse) performs T1, T2 and PD mapping in a single 3D acquisition, and can generate synthetic images with tunable contrast. It was initially developed for cardiac imaging and more recently for brain. However, extending multi-parameter mapping to general body applications has been challenging due to partial volume effects of fat. When two tissue types are present in the same voxel, their independent T1 and T2 values are difficult to distinguish. In this work, we combined a Dixon fat-water method with 3D QALAS to enable 3D, water-only multi-parameter mapping of human breast.Methods
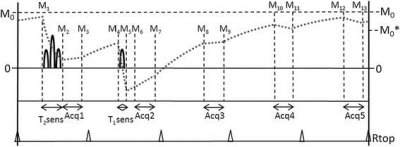
The 3D QALAS sequence was implemented for untriggered acquisitions in the head and body. This sequence consists of a 5 phases of varying contrasts acquired with 3D gradient echo readouts in a single cycled acquisition. A T2 prep sequence precedes the first phase, followed by an inversion prep pulse and 4 delayed phases (figure 1). One cycle would acquire one segment of Cartesian k-space for each of the 5 phases with spoiled gradient echo readouts, such that repeating the cycle would fill all segments of k-space for 5 image sets. The T2 prep time and the initial delay after the inversion prep pulse were both set at 90 msec. The sequence timing was arranged such that the 5 phases of the acquisition were equally spaced in time at 0.91 sec, mimicking a triggered cardiac acquisition of approximately 66 beats/min. The sequence was then further modified to enable a bipolar dualecho readout to acquire data at approximately the in-phase and out-of-phase echo times of water and fat, producing 10 raw image sets in all. All 5 pairs of complex in- and out-of-phase data were processed with a flexible TE fat-water separation technique with joint estimation [2,3] to produce 5 water-only and 5 fat-only image sets. A female subject with breast cancer was imaged with the 3D QALAS Dixon sequence on a 3T wide bore scanner (MR750W, GE Healthcare, Waukesha, WI) using and 8-channel breast coil. Sequence parameters for breast imaging were: FOV = 34.0 cm, matrix = 256x192, slice locations = 88, thickness = 3.0 mm, flip = 4°, TR = 4.2, TE = 1.3 and 2.5 msec, views per segment=100, ARC acceleration factor = 2, total scan time = 3:56. Subject was also scanned with a 2D multiecho multidelay (MDME) technique [4] without fat suppression or fat-water separation. Quantitative T1, T2, and PD maps were reconstructed from the water-only 3D QALAS and unsuppressed 2D MDME images sets using a research version of SyMRI (SyntheticMR, Linkoping, Sweden).Results
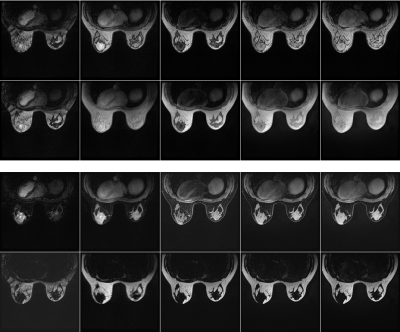
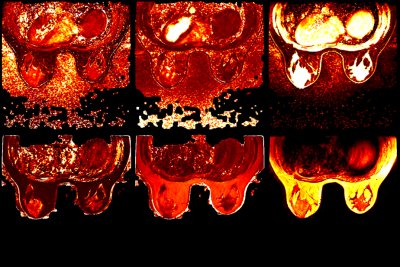
Fat-water separation was successfully performed on all 5 raw image pairs, with no swaps or misclassification observed in the water- or fat-only images. No shading of the T1 and T2 maps was observed over the entire volume. One lesion surrounded by fat was markedly less visible in the maps generated from the 2D non-suppressed acquisition when compared to those generated by 3D QALAS with Dixon (Fig. 3).Discussion and Conclusion
We demonstrated the feasibility of 3D multi-parameter mapping with Dixon fat-water separation for breast imaging. Although the smaller voxel size of the 3D acquisition could reduce some of the partial volume effects of fat, separation of the two species is critical for accurate quantitation of water tissue. The phase evolution of fat relative to water over multiple echo times remains the most reliable basis for fat-water separation, as T1 and T2 values alone are typically not sufficient to distinguish the two species in the same voxel without some tenuous assumptions. The adapted flexible TE algorithm improves robustness further by incorporating information from all 5 pairs of in- and out-of-phase echoes for field estimation. Though the inversion pulse potentially changes the polarity of the spins at subsequent phases, it did not affect the result of the fat-water separation algorithm. While the dualecho acquisition is very efficient, a slight chemical shift induced edge artifact was observed in the parameter maps. While a B1 measurement was not built into the 3D technique, the effects of B1 inhomogeneity were relatively small, partially due to the low flip angle (4 degrees) required for the gradient echo acquisition. The technique also does not require modeling of the slice profile for much of the center portion of the slab. Thus 3D QALAS with Dixon fat-water separation is an excellent method for rapid multi-parameter quantification and synthetic fat-suppressed imaging in the breast, and potentially could be extended to other applications such as body or spine imaging.Acknowledgements
Research support was provided in part by GE Healthcare.References
1. Kvernby S, Warntjes MJ, Haraldsson H, Carlhäll CJ, Engvall J, Ebbers T. Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS. J Cardiovasc Magn Reson. 2014; 16:102.
2. Ma J, Son JB, and Hazle JD. An improved region growing algorithm for phase correction in MRI. Magn Reson in Med. 2015;76:519-529.
3. Son JB, Hwang KP, Madewell JE, Bayram E, Hazle JD, Low RN, and Ma J. A Flexible Fast Spin Echo Triple-Echo Dixon Technique. Magn Reson in Med. 2017;77:1049-1057.
4. Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn Reson Med. 2008 ; 60:320-9.
Figures