5621
Liquid Crystal Magnetic Resonance Visible Thermometer1Physical Measurement Laboratory, National Institute of Standards and Technology, Boulder, CO, United States, 2High Precision Devices, Boulder, CO, United States
Synopsis
A magnetic resonance thermometer with liquid crystal compartments was designed and prototyped to provide a method of non-invasively measuring temperature when imaging MR phantoms. We successfully demonstrate the liquid crystal MR visible (LC-MRV) thermometer using different, fast, sequences on both pre-clinical and clinical systems. The LC-MRV thermometer is designed to exhibit rapid cholesteric to isotropic transitions in the room temperature range spanning 15.0°C to 25.0°C in 0.1°C to 1.0°C increments. It is demonstrated that the LC-MRV thermometer is resilient to large magnetic fields and therefore variations in the magnetic field, providing an accurate determination of the temperature during quality control scans where bore temperatures can vary day-to-day and scanner-to-scanner. It also provides a rapid assessment of temperature changes over the duration of the scan.
Introduction
MR phantoms contain solutions with exact and traceable values of particular MR biomarkers, e.g. T1 and T2 relaxation time or apparent diffusion coefficient (ADC). It is well known that the values can exhibit temperature dependence and variations in MRI bore temperatures can span from 15.0°C to 25.0°C rendering the quantitative nature of the phantom irrelevant if not accurately corrected for temperature.1 There are invasive ways to measure the temperature of MRI phantoms using digital, alcohol, or fiber optic thermometers. However, a non-invasive, MR visible thermometer that can be read using typical imaging protocols is preferred. A well-known experimental method to determine temperature is to image the chemical shift observed between two different materials or resonances in a molecule. However, variations in the magnetic field strength will introduce errors in the chemical shift based thermometer, making it inadequate for traceable standards2,3. Liquid crystals have previously been explored as an option for in vivo MR thermometry4.
Here we describe a cholesteric liquid crystal magnetic resonance visible (LC-MRV) thermometer than can be placed in an MR phantom and easily imaged during routine scanning.
Methods
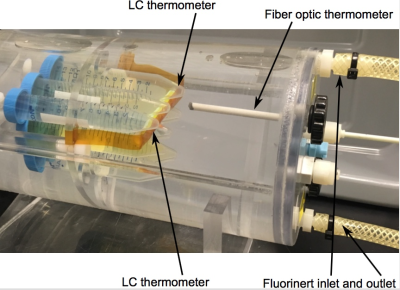
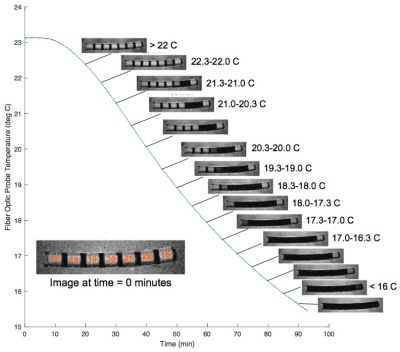
The LC-MRV thermometer is designed to exhibit rapid cholesteric to isotropic transitions in the room temperature range spanning 15.0°C to 25.0°C in 0.1°C to 1.0°C increments. The liquid crystals reside in compartments made of MRI compatible plastics that are chemically inert to the cholesteric liquid crystals (Fig. 1). The compartment dimensions are sized to allow for reasonable MR signal, which presents as a bright spot when the liquid crystals are in the isotropic state and a dark spot when the liquid crystals are in the cholesteric state (Fig. 2).
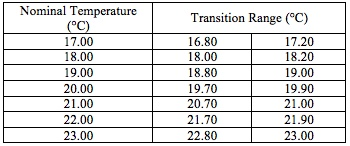
The transition temperatures for the LC-MRV thermometer were assessed visually, and the temperature was measured with a high-accuracy, digital thermometer (0.1-0.2°C accuracy) when the isotropic to cholesteric transition began and was completed. These temperatures are given in Fig. 3/Table 1.
To demonstrate the use and accuracy of the LC thermometer, we used several different MR sequences on both a pre-clinical MR system and a clinical system both at 3 T:
Pre-clinical GRE: flip angle 70°, TR 200 ms, TE 5.00 ms, 0.55 mm2
Pre-clinical spin-echo: flip angle 90°, TR 20.10 ms, TE 13.82 ms, 0.55 mm2
Pre-clinical spoiled gradient echo (SPGR): flip angle 20°, TR 60.48 ms, TE 5.00 ms, 1.17 mm2
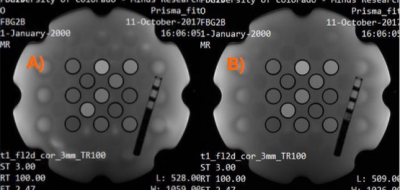
Clinical SPGR: flip angle 70°, TR 200 ms, TE 2.47 ms, 0.625 mm2
Pre-clinical measurements were conducted in a temperature-controlled chamber (Fig. 1). For the experiment, the chamber was equilibrated at 23.1°C; then, the outer shell (Fluorinert) temperature was driven to 10.0°C using a programmable, temperature-controlled, circulating bath. Over the ninety-minute experiment, the water bath temperature in the chamber, measured by fiber optic probe, dropped to below 16.0°C. At five-minute increments, GRE and spin-echo images were obtained (Fig. 2).
Results and Discussion
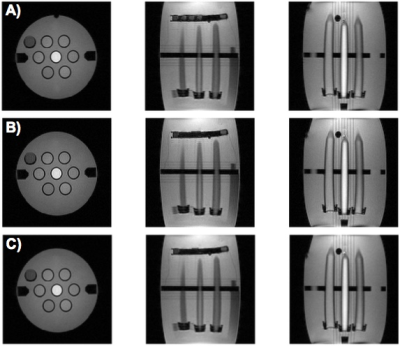
The LC-MRV thermometer was constructed as described and successfully imaged in pre-clinical (Fig. 2 and 4) and clinical MR systems (Fig. 5). In the experiment described in Fig. 2, there is a difference between the FO and LC-MRV measurements, likely due to thermal gradients in the water bath, since the two thermometers were separated by 2-3 cm. The images obtained in Fig. 4, when the chamber had reached equilibrium, show agreement between the FO and LC-MRV measurements.
The LC-MRV thermometer was included in an MR phantom during routine scanning on a clinical system (Fig. 5). The initial temperature measurements are different, most likely because the scan room was significantly warmer than the phantom, and thermal changes were already underway during the time differential between when the digital thermometer measurement was made and 5-10 minutes later when the phantom was loaded and scanned for the LC-MRV measurement. The SPGR images visually show the temperature rise from initial to final scan (scan session roughly one hour).
Conclusion
We successfully demonstrate a non-invasive MR visible thermometer that is easily scanned on both pre-clinical and clinical systems using almost any sequence configuration since it relies on relative signal intensity. It is demonstrated that the LC-MRV thermometer is resilient to large magnetic fields, and therefore variations in the magnetic field, providing an accurate determination of the temperature during quality control scans where bore temperatures can vary day-to-day and scanner-to-scanner. Additionally, the LC-MRV thermometer can be easily incorporated into MR phantoms, and the LC-MRV thermometer design has a rapid response to changes in temperature.Acknowledgements
High Precision Devices received support from a NIST SBIR award.References
1. P. Baron, R. Deckers, S.M. Sprinkhuizen, L.G. Merckel, R.L. Bleys, C.J. Bakker, and L.W. Bartels. Measurement of the T1 and T2 Temperature dependence of human breast adipose tissue. Proc. Intl. Soc. Mag. Reson. Med, 2011. p. 1772.
2. M.L. Kaplan, F.A. Bovey, and H.N. Cheng. Simplified Method of Calibrating Thermometric Nuclear Magnetic Resonance Standards. Anal. Chem, 1975. 47(9): p1703-1705. N. Hedin and I. Furo. Temperature imaging by 1H NMR and suppression of convection in NMR probes. J. Magn. Res., 1997. 131: p.126-130
3. S.M. Sprinkhuizen, C.J.G. Bakker, and L.W. Bartels. Absolute MR thermometry using time-domain analysis of Multi-Gradient-Echo magnitude images. Magn. Reso. Med., 2010. 64: p. 239-248.
4. K.J. Franklin et al. Encapsulated liquid crystals as probes for remote thermometry. Int. J. Hyperthermia,1992. 8(2): p. 253-262.
Figures