5607
Comparison of two alternative sequences for human in-vivo brain MR Current Density Imaging (MRCDI)1Center for Magnetic Resonance, DTU Elektro, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital, Hvidovre, Denmark, 3Department of Neurology, Copenhagen University Hospital, Bispebjerg, Denmark, 4High-Field Magnetic Resonance Center, Max-Planck-Institute for Biological Cybernetics, Tübingen, Germany, 5German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 6Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany
Synopsis
MRCDI is a novel technique, utilizing different phase-sensitive MR methods for non-invasive measurements of weak currents in the human body, which is important in several neuroscience applications. Here, we compare the in-vivo performance of two different MR methods, multi-echo spin echo (MESE) and steady-state free precession free induction decay (SSFP-FID), with single- vs. multi-gradient-echo readouts. We demonstrate that multi-gradient-echo readouts improve both methods. We validate the linear dependence of the measured current-induced magnetic field on the injected current strength for both methods, and propose the more efficient SSFP-FID method as being well suited for highly sensitive single-slice human in-vivo MRCDI.
Introduction
Accurate measurements of the current flow distributions, caused by neural or external sources, in the human brain in-vivo is important in several neuroscience applications. MRCDI is an emerging technique, which combines MR methods with alternating currents for in-vivo current flow measurements in the human brain at high resolution (1). The efficiency of the technique depends on the used MR method. MESE is a technique robust to field inhomogeneities and provides high signal-to-noise-ratio (SNR). On the other hand, SSFP-FID is more sensitive to current-induced phase, but more vulnerable to field inhomogeneities. Both methods are promising for in-vivo human brain MRCDI. We previously performed a thorough sensitivity comparison of MESE and SSFP-FID MRCDI in phantoms (2). However, in-vivo robustness of the methods to physiological noise was not analyzed. Here we compare MESE and SSFP-FID in-vivo, and propose SSFP-FID as a better alternative for single-slice human in-vivo brain MRCDI applications.Methods
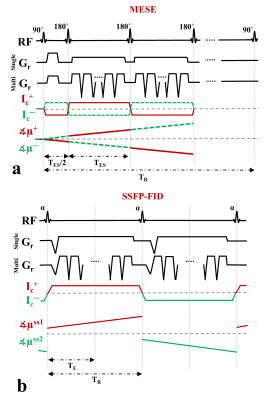
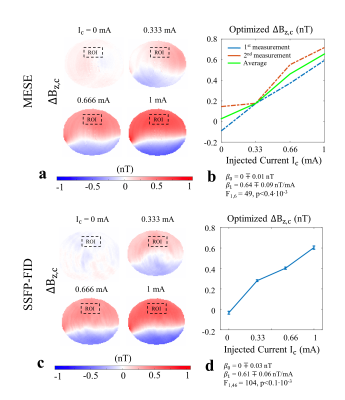
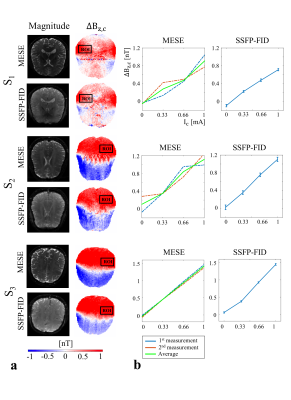
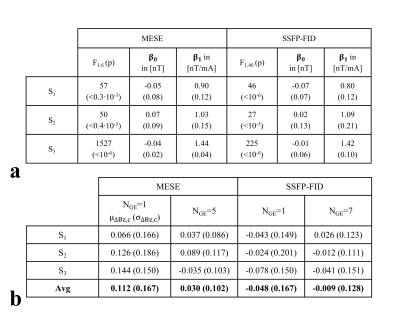
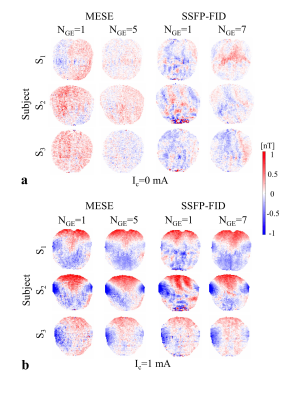
In MRCDI, weak alternating tissue currents, which are synchronized with an MR sequence, create a magnetic field component of the current-induced magnetic field ∆Bz,c parallel to the scanner field. This causes small shifts in the precession frequency of the magnetization. These modulate the phase of the MR signal, proportional to ∆Bz,c, and allows measurement of ∆Bz,c and calculations of the current flow distribution. Here, we employed MESE (Fig.1a) and SSFP-FID (Fig.1b) and used previously optimized sequence parameters (2) for human in-vivo MRCDI. First, we validated both methods by checking the linear dependence of the measured ∆Bz,c on the current strength Ic. A cable was wrapped around a spherical phantom (16 cm in diameter) with similar relaxation parameters as brain tissue. Using both methods, ∆Bz,c was measured for Ic = [0, 0.33, 0.66, 1] mA. The MESE measurements were performed with image matrix 112x90, voxel size 2x2x3mm3, number of spin-echo NSE=3, gradient-echo NGE=5, bandwidth BW=103.6 Hz/pixel, echo spacing TES=60 ms, and repetition time TR=1.5 s. The measurement was repeated Nmeas=2 times. The SSFP-FID measurements were performed with image matrix 112x90, voxel size 2x2x3mm3, tip angle α=30˚, NGE=7, BW=75 Hz/pix, TR=120 ms, and Nmeas=12. The total scan times were close to ~9mins and ~4.5 mins for MESE and SSFP-FID, respectively. The same experiments were also repeated in-vivo in three participants. The average ∆Bz,c values were extracted, and a linear regression model fitted to the measurements. As a last step, single- and multi-gradient-echo readout performances of the methods were tested in three subjects. MESE (NGE=1/5, BW = 19.2/103.6 Hz/pixel) and SSFP-FID (NGE=1/7, BW = 12/75 Hz/pixel) experiments were repeated with Ic= 1 mA and without current injection. Gaussian distributions were fitted to the ∆Bz,c distributions of the control experiments without Ic and the methods were compared in terms of the mean shifts and the standard deviations. In the experiments, the current waveform was generated using an arbitrary waveform generator (33500B, KEYSIGHT Technologies, California, United States), amplified via an MR-conditional transcranial brain stimulator (DC-STIMULATOR PLUS, neuroConn GmbH, Germany), and injected via rubber electrodes attached to the scalp (close to the temporo-parietal junction).Results and Discussion
The ∆Bz,c measurements for MESE and SSFP-FID show a linear dependence on the injected current strength in both the phantom (Fig. 2) and in-vivo (Fig. 3). The intercepts β0 are close to zero, and the estimated slopes β1 found similar in both methods (Fig. 4a). The results of linear regression analyses are highly significant. The use of multi-gradient-echo readouts improve the quality of the measured ∆Bz,c images with and without Ic. The ghosting-like artifacts disappear (Fig. 5). The results without current injection show that the mean shifts are close to zero and the standard deviations are effectively reduced by means of multi-gradient-echo readouts (Fig. 4b). MESE exhibits a lower noise floor compared to SSFP-FID in two out of three subjects. Both MESE (total scan time Ttot~9 mins) and SSFP-FID (Ttot~4.5 mins) have similarly low noise floors with standard deviations of on average ~0.1 nT.Conclusion
Both MESE and SSFP-FID methods are validated in phantom and in-vivo experiments. The use of multi-gradient-echo readout provides better results, as systematic averaging of multi-echoes reduces detrimental effects (e.g. blood flow and motion). Both methods provide sufficiently low noise floors for human brain MRCDI. The higher number of measurements used in SSFP-FID may allow a better removal of physiological noise and outliers in the future, which may make the method the better choice for single-slice in-vivo MRCDI. However, MESE can still outperform SSFP-FID in multi-slice MRCDI, as it allows acquiring few slices without losing sensitivity (2).Acknowledgements
The project is supported by Lundbeck foundation with grant number R118-A11308.References
1. Scott GC, Joy MLG, Armstrong RL, Henkelman RM. Sensitivity of magnetic-resonance current-density imaging. J. Magn. Reson. 1992;97:235–254.
2. Göksu C, Scheffler K, Ehses P, Hanson LG, Thielscher A. Sensitivity Analysis of Magnetic Field Measurements for Magnetic Resonance Electrical Impedance Tomography (MREIT). Magn. Reson. Med. 2017. doi: 10.1002/mrm.26727.
Figures