5600
Power-Law Multi-Frequency MR Elastography of the Human Brain with Super-resolution Imaging via Non-Linear Inversion1Département de génie mécanique, Université de Sherbrooke, Sherbrooke, QC, Canada, 2SoundBite Medical Solutions Inc., Sherbrooke, QC, Canada, 3Department of Radiology, Charité-Universitätsmedizin, Berlin, Germany, 4Thayer School of Engineering, Dartmouth College, Hanover, NH, United States, 5Department of Radiology, Dartmouth-Hitchcock Medical Center, Lebanon, NH, United States
Synopsis
This abstract presents a non-linear inversion based power-law multi-frequency MR Elastography reconstruction, capable of reconstructing images at resolutions finer than the acquired displacement data. Reconstructed power-law parameters are compared with those obtained by logarithmic regression based on mono-frequency reconstructions of the same data.
INTRODUCTION
The interest in viscoelastic parameters obtained from MR Elastography has grown continuously since the development of the method, and preliminary results have indicated the potential of these damping properties as biomarkers for a number of targets, including the liver and the brain1. Viscous property characterization has now begun to seek to identify the parameters describing the spectral tissue response across a range of frequencies through power-law (PL) models that can be related to multi-scale effects in both scattering2 and fluid-solid interactions3. This work presents the development of a multi-frequency reconstruction method based on a power-law model (PL-MF MRE) for the storage and loss modulus. The PL-MF MRE reconstruction method is presented via in-vivo data in the human brain and the resulting PL parameters are compared to values obtained from regression of mon-frequency reconstructions.METHODS
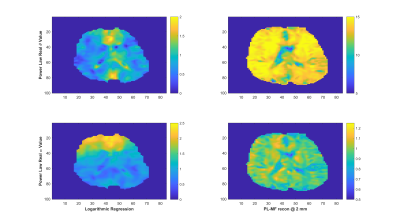
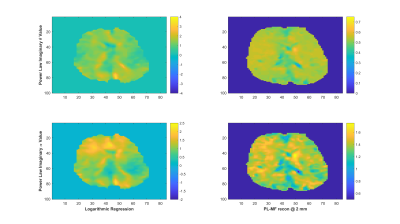
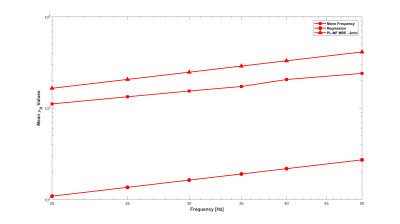
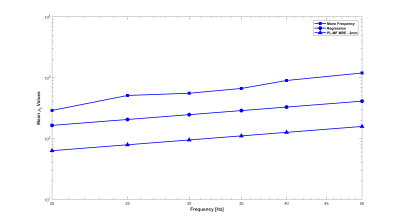
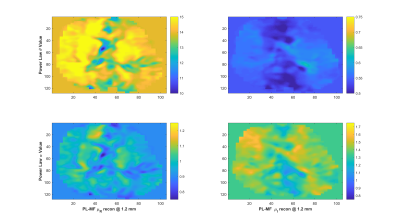
Experiments were conducted on a 1.5-T Siemens MAGNETOM Sonata using single-shot spin-echo EPI with trapezoidal flow-compensated MEGs in all three directions4. The actuator employed was similar to the head rocker system described by Sack et al5., with the setup detailed by Guo et al6. Full 3D displacement fields at 15, 20, 25, 30, 35, 40 and 50 Hz were obtained in healthy volunteers. Guideline Values: Guidelines for the PL parameters for the viscoelastic material properties were established via mono-frequency reconstructions using non-linear inversion (NLI)7. The resulting distributions for μR and μI were fit to independent PL models, μR = θR·ωαR and μI = θI·ωαI, through a logarithmic regression at each voxel8, providing distributions and volumetric means of θR, αR, θI and αI, as well as the RMS error for the regression fit. PL-MF MRE: The same displacement fields were then provided to a NLI based PL-MF MRE reconstruction algorithm, based on finite element methods and adjoint-gradient based optimization9 of the objective function, $$\Phi=\frac{1}{2}\sum_{i=1}^{N_{freq}}\|u_c(\omega_i)-u_m^i\|.^2$$ Total Variation (TV) regularization was applied to counteract decreased stability of the inversion given the strict application of the PL model. The results from the PL-MF MRE reconstruction are the real and imaginary shear modulus PL parameters, θR, αR, θI and αI, for the frequency range provided. The distributions and volumetric averages of these parameters were compared with the guideline values obtained by logarithmic regression. Super-resolution MRE: While MRE is technically ‘super-resolution’ by default, due to the shear wavelength being longer than typical imaging voxel resolutions, it is possible to reconstruct material properties at resolutions finer than the imaging resolution, provided sufficient data10. Here, the presence of 6 measurements (real and imaginary displacements in 3 directions) at each frequency, versus the 4 parameters to be reconstructed, (θR, αR, θI, αI), permitted reconstruction at finer resolution than the measured displacement data. For the finite element implementation used for the PL-MF MRE reconstruction, the limiting factor for the reconstruction resolution was the resolution of the Gauss points used to integrate the state equations for the calculated displacements. For the 2 mm isotropic voxels used here, material parameter resolutions smaller than 1.2 mm would generate cases where the resulting material property had no influence on the displacement values, thus limiting reconstruction resolution unless the measured displacements were interpolated onto a finer resolution mesh, which was not done in this case. Super-resolution distributions of θR, αR, θI and αI were calculated on 1.2 mm voxels using the same PL-MF MRE method used for 2 mm voxels.RESULTS
As reported in Testu et al8., mono-frequency reconstructed properties were generally well described by a power-law model, except in the region of the falx cerebri. The TV stabilized PL-MF MRE reconstruction provided similar PL parameters to the regression results, with image definition clearly improved through the incorporation of multiple displacement fields into the NLI algorithm. Figures 1 and 2 compare the guideline values for the PL parameters and those from the PL-MF MRE reconstruction for the real and imaginary shear modulus, respectively. Figures 3 and 4 show the resulting fit to the mono-frequency real and imaginary shear modulus values, respectively. Finally, Figure 5 shows the super-resolution PL parameters at 1.2 mm, reconstructed via PL-MF MRE. In general, guideline PL parameter values are in good agreement with those obtained by PL-MF MRE, with the largest disagreements coming in regions of high RMS error for the logarithmic regression.CONCLUSIONS
This preliminary demonstration of a PL-MF MRE method able to match the PL parameters obtained from regression analysis of mono-frequency reconstruction results provides an important step toward accurate and verifiable quantification of the dispersive, multi-scale nature of soft-tissue.Acknowledgements
This work was supported in part by NIH Grants R01-EB018230 awarded by the National Institute of Biomedical Imaging Bioengineering and R01-AA023684 awarded by the National Institute on Alcohol Abuse and Alcoholism. Financial support of the Bundesministerium fr Bildung und Forschung (BMBF 01GQ1408) is gratefully acknowledged. EEWVH acknowledges financial support from NSERC (Discovery Grant #435399-2013) for this work and is a member of the FRQ-S-funded Centre de recherche du Centre hospitalier universitaire de Sherbrooke (CR-CHUS).References
1. Klatt D, Hamhaber U, Asbach P, Braun J, Sack I. Noninvasive assessment of the rheological behavior of human organs using multifrequency MR elastography: a study of brain and liver viscoelasticity. Physics in Medicine and Biology. 2007; 52(24): 7281–7294.
2. Sinkus R, Siegmann K, Xydeas T, et al. MR elastography of breast lesions: Understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magnetic Resonance in Medicine. 2007; 58(6): 1135-1144.
3. Parker K. A microchannel flow model for soft tissue elasticity. Physics in Medicine and Biology. 2014; 59(15): 4443.
4. Dittmann F, Tzschätzsch H, Hirsch S, et al. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magnetic Resonance in Medicine. 2016; 76(4): 1116-1126.
5. Sack I, Beierbach B, Hamhaber U, et al. Non-invasive measurement of brain viscoelasticity using magnetic resonance Elastography. NMR in BioMedicine. 2008; 21(3): 265-271.
6. Guo J, Hirsch S, Fehlner A, et al. Towards an Elastographic Atlas of Brain Anatomy. PLoS ONE. 2013; 8(8): e71807.
7. McGarry M, Johnson C, Sutton B, et al. Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE transactions on medical imaging. 2013; 32(10): 1901-1909.
8. Testu J, McGarry M, Dittmann F, et al. Viscoelastic power law parameters of in vivo human brain estimated by MR Elastography. Journal of the Mechanical Behavior of Biomedical Materials. 2017; 74: 333-341.
9. Tan L, McGarry M, Van Houten E, et al. Gradient-Based Optimization for Poroelastic and Viscoelastic MR Elastography. IEEE transactions on medical imaging. 2017; 36(1): 236-250.
10. Perrins M, Barnhill E, Kennedy P, et al. Super-Resolution Magnetic Resonance Elastography (SR-MRE) of Exercise Induced Muscle Damage (EIMD). Proceedings of the 24th Annual Meeting of the ISMRM. 2016; 0752.
Figures