5582
Interventional MR Elastography and thermometry using simultaneous image refocusing (SIR) for multislice monitoring of thermal therapies1ICube CNRS / University of Strasbourg, Strasbourg, France, 2Department of Interventional Imaging, Strasbourg university hospital, Strasbourg, France
Synopsis
Interventional MR Elastography (MRE) and thermometry (PRF) using the Simultaneous Image Refocusing (SIR) technique is proposed in order to monitor changes in temperature and biomechanical properties during thermal ablations, in multiple contiguous slices. Compared to the conventional GRE-MRE sequence, two slices are acquired in each TR, with a single motion sensitizing gradient and similar fractional motion encoding. Elasticity maps obtained with SIR-GRE MRE are validated against reference single slice GRE-MRE. Preliminary experiments show the potential of contiguous multislice acquisition to monitor the volumetric extent of the heated area obtained with High Intensity Focused Ultrasound (HIFU) in a phantom.
Introduction
Interventional MR Elastography (MRE) has been developed to monitor changes in biomechanical properties induced by thermal ablations1. Based on a GRE sequence, it allows deriving both elasticity and thermometry maps simultaneously in a single slice1-2 at a typical update rate of 2s. However, real-time monitoring of thermal ablations requires the acquisition of multiple contiguous slices in order to spatially cover the ablation area, while preserving sufficient temporal resolution. This paper proposes the use of the Simultaneous Image Refocusing (SIR) technique3 for the simultaneous MRE encoding and acquisition of 2 slices in each single acquisition.Methods
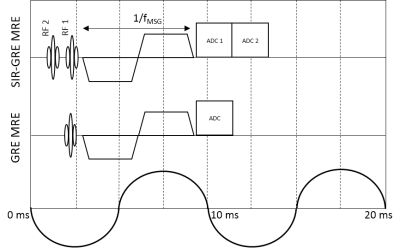
As shown in Fig. 1, the SIR-GRE MRE sequence acquires two sequential echoes in a single TR, created with two RF pulses in combination with two pre-dephasing readout gradients. Hence two non-contiguous slices are acquired per TR with a single motion sensitizing gradient (MSG), each slice having its specific TE3. It is possible to acquire the echoes for 2 slices with TR = 2 mechanical periods (Texc), similar to the typical GRE MRE protocol. All experiments are conducted in a 1.5 T MRI scanner (MAGNETOM Aera, Siemens, Germany). Pneumatic excitation is used for wave generation within the phantoms. Elastograms are reconstructed by using a local frequency estimation (LFE)-based algorithm. Motion is encoded through slice. Proton Resonance Frequency (PRF) shift thermometry is used to evaluate temperature variations from the MRE dataset1-2. The first experiment aims at comparing the proposed SIR-GRE MRE sequence to a regular single slice GRE MRE sequence. It is performed on a 7% gelatin phantom with a 9% gelatin cylindrical inclusion. Slices 1 and 2 are axial to the inclusion main axis, with a slice gap of 7 mm. Relevant imaging parameters common to both sequences are: mechanical excitation frequency 100 Hz, MSG frequency 122 Hz, 4 phase offsets for temporal Fourier transform, TR 20 ms, FOV 250 mm x 250 mm, thickness 7 mm, matrix 128 x 128, flip angle 10° and bandwidth 1000 Hz/pixel. For the proposed SIR-GRE MRE sequence, TE1/TE2 = 10.2/12.6 ms, and the acquisition time for 2 slices is 2560 ms. For the reference GRE-MRE sequence, TE = 10.2ms, and the acquisition time per slice is 2560 ms, i.e. the acquisition time for 2 slices is 5120 ms. The second experiment is performed in a 7% gelatin phantom containing bovine muscle. HIFU ablation starts 30s after the SIR-GRE MRE acquisition begins. It lasts 60s at an acoustic power of 30 W, with the focal spot being positioned at the interface between tissue and gelatin. Two acquisitions of two slices (slice gap 100% between 2 simultaneously acquired slices) are interleaved with no space between slices. Slices 1 to 4 cover a volume going from pure gelatin to pure tissue, centered on the interface between gelatin and tissue. Other imaging parameters are equal to those used in experiment 1 with the SIR-GRE MRE sequence, except that 3 phase offsets are used for higher update rate.Results
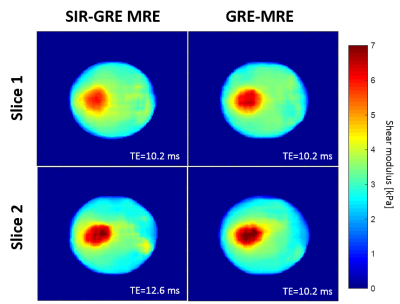
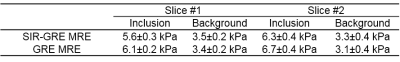
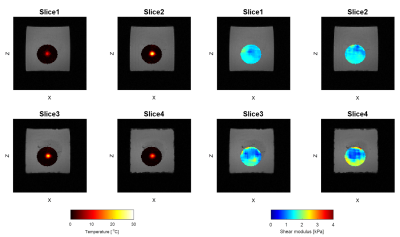
Means and standard deviations calculated respectively in the inclusion and the background for both slices in experiment 1 are shown Table 1. Values of shear moduli found with the SIR-GRE MRE sequence were found to be highly similar to those obtained with the reference GRE-MRE. Figure 3 illustrates temperature and elasticity maps on the 4 contiguous slices 100 seconds after the end of heating. Differences in temperature elevation across the 4 slices are clearly visible, as well as differences in elasticity due to the fact that these 4 slices are located around the tissue/gelatin interface.Discussion
Simultaneous MRE/MR thermometry aims at improving the monitoring of thermal therapies by providing two complementary biomarkers, namely the changes in elasticity and temperature. Thermal ablations are volumetric, hence their monitoring by MRI should be done with multislice acquisitions. As shown in Fig. 3, temperature estimates, and therefore thermal dose to a greater extent, differ significantly from one slice to another. However, acquiring multiple slices means diminishing the temporal resolution. In GRE MRE, the MSG duration limits the minimum TR that can be used. The straightforward acceleration method is to use strong fractional encoding so that the TR can be equal to a single mechanical period, instead of two. However, this also implies lower wave encoding. The SIR-GRE MRE sequence offers an equivalent acceleration factor for two slices, with similar elasticity values as the reference single slice GRE sequence.Conclusion
The SIR-GRE MRE sequence provides stiffness values and temperature shifts simultaneously in multiple slices, while minimizing the loss in terms of temporal resolution.Acknowledgements
This study was partly funded by French excellence program Labex CAMI (ANR-11-LABX-0004).References
1. Corbin N, Vappou J, Breton E, et al. Interventional MR elastography for MRI-guided percutaneous. Magn Reson Med. 2015;75(3):1110-1118.
2. Le Y, Glaser K, Rouviere O, et al. Feasibility of simultaneous temperature and tissue stiffness detection by MRE. Magn Reson Med. 2006;55:700-705.
3. Feinberg DA, Reese TG, Wedden VJ. Simultanous echo refocusing in EPI. Magn Reson Med. 2002;48(1):1-5.
Figures