5575
Coupled temporal variation and global functional connectivity density of spontaneous brain activity in self-hypnosis for respiratory motion control1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
This study aims to explore the correlative relationship between temporal variation and signal synchronization of spontaneous brain activity in self-hypnosis for respiratory motion control and relaxation. A resting-state fMRI was employed to an intra-subject of 15 hypnotist volunteers in rest state and self-hypnosis state to explore the inter-state difference of correlation within four conventional resting-state networks. The results demonstrated that coupled temporal variation and signal synchronization of brain activity in self-hypnosis. It provides neural implications of self-hypnosis, a psychological technology that can generate positively psychological and physiological effects, in controllable self-regulation, which is beneficial to cancer patients during radiotherapy.
INTRODUCTION
As reported in our previous study,1 hypnosis is proposed as an effectively psychological
technique in respiratory motion control, which contributes to the restriction
and stability of tumor motion (especially for chest and abdominal tumors)
during radiotherapy. However, the underlying neural mechanism remains
mysterious. This paper aims to explore the correlative relationship between
temporal variation and signal synchronization of spontaneous brain activity in
self-hypnosis for respiratory motion control and relaxation.
METHODS
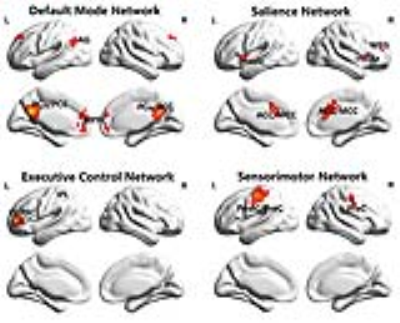
An intra-subject design of 15 healthy hypnotist volunteers was employed to a self-hypnosis experiment in a 3.0T SIEMENS magnetic resonance imaging (MRI) machine. Volunteers were tested in two states, rest state (RS) and self-hypnosis state (SHS). The functional image and T1 image of each volunteer’s brain were obtained for both two states. A resting-state functional MRI was applied to explore the correlation of temporal variation and signal synchronization of blood oxygenation level dependent signal within four conventional resting-state networks. Temporal variation of brain signal was detected by amplitude of low frequency of fluctuation (ALFF) (0.01-0.08Hz)2 and fractional ALFF (fALFF) (0.01-0.08Hz)3. Signal synchronization was measured by regional homogeneity (ReHo)4 and degree of centrality (DC) (threshold of 0.25)5, reflecting local and global functional connectivity respectively. Four networks, default mode network, salience network, executive control network and sensorimotor network were identified by seed-wise functional connectivity. The seeds of these networks were selected according to priori study6 as following: precuneus/posterior cingulate cortex for DMN7, dorsal anterior cingulate cortex for SN8, left dorsolateral prefrontal cortex for ECN8, left central sulcus for SMN9. Moreover, a set of intrinsic networks template nodes10 were applied to examine the robustness of the results. Additionally, a range thresholds (from 0.25 to 0.75) of DC were further calculated to explore the stability of correlation results. Both averaged level and group level of the correlation were measured.RESULTS
The four large scale networks identified by seed-wise functional connectivity in this study (Figure 1) were mostly in keeping with previous study6. Within the identified networks, enhanced correlations were observed between temporal variation and signal synchronization (Figure 2C) in SHS. The enhanced results were robust in response to a range threshold of DC (Figure 2D). Most importantly, coupled temporal variation (ALFF/fALFF) and global functional connectivity (DC) were demonstrated (ALFF-DC: R=0.09/0.73, p=0.7752/0.0068 in RS/SHS; fALFF-DC: R=0.26/0.79, p=0.4056/0.0023 in RS/SHS) (Figure 2A,2B). Moreover, coupled ALFF and DC (R=0.34/0.70, p=0.0593/p<0.0001 in RS/SHS) was also demonstrated in another set of template (Figure 3A,3B). The results were overall consistent in the identified template and prior template (Figure 2, Figure 3).DISCUSSION
Comparing to the results in RS, coupled correlation of temporal variation and global functional connectivity density (DC) of brain activity was observed in SHS. It suggests that self-hypnosis may rise a whole cross-brain of intra-connection when regulating mental state and physical performance (respiratory motion) personally. Interestingly, our results are contrary to the findings of unconscious state that demonstrated decoupled temporal variation and signal synchronization6. Hypnosis is proposed to be an enhanced attentional state11. Self-hypnosis shares something in common with hypnosis guided by hypnotist. The enhanced and coupled correlations among spontaneous brain activities indicates a more physically and mentally controllable self-regulation in self-hypnosis, which is beneficial to cancer patients during radiotherapy.CONCLUSION
Our findings provide implications for the neural basis of self-hypnosis for respiratory motion control as an enhanced attention state. Hypnosis is a psychological technology that can generate positively psychological and physiological effects. Clinical trials on cancer patients during radiotherapy are necessary to further examine the therein mechanisms.Acknowledgements
This work is supported in part by grants from Shenzhen Key Technical Research Project (JSGG20160229203812944), National Key Research and Develop Program of China (2016YFC0105102), National Science Foundation of Guangdong (2014A030312006) and Beijing Center for Mathematics and Information Interdisciplinary Sciences.References
1. Li R, Deng J, Xie Y. Control of respiratory motion by hypnosis intervention during radiotherapy of lung cancer i. Biomed Res Int. 2013;2013:574934.
2. Zang Y, He Y, Zhu C, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83-91.
3. Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172(1):137-141.
4. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394-400.
5. Buckner RL, Sepulcre J, Talukdar T, et al. Cortical Hubs Revealed by Intrinsic Functional Connectivity: Mapping, Assessment of Stability, and Relation to Alzheimer’s Disease. J Neurosci. 2009;29(6):1860-1873.
6. Huang Z, Zhang J, Wu J, et al. Decoupled temporal variability and signal synchronization of spontaneous brain activity in loss of consciousness: An fMRI study in anesthesia. Neuroimage. 2016;124:693-703.
7. Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the Functional Heterogeneity of the Default Mode Network Using Coordinate-Based Meta-Analytic Modeling. J Neurosci. 2009;29(46):14496-14505.
8. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J Neurosci. 2007;27(9):2349-2356.
9. de Pasquale F, Della Penna S, Snyder AZ, et al. A Cortical Core for Dynamic Integration of Functional Networks in the Resting Human Brain. Neuron. 2012;74(4):753-764.
10. Shackman AJ, Salomons T V., Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154-167.
11. Jiang H, White MP, Greicius MD, Waelde LC, Spiegel D. Brain activity and functional connectivity associated with hypnosis. Cereb Cortex. 2017;27(8):4083-4093.
Figures