5574
Semantic verbal memory outcome in TLE and ETLE patients after surgery using fMRI1Neurology, AIIMS, New Delhi, India, 2NMR, AIIMS, New Delhi, India, 3Neurosurgery, AIIMS, New Delhi, India, 4Neuropsychology, AIIMS, New Delhi, India
Synopsis
Memory can be broadly classified as sensory (process > 1 second), short term (>1 minute) and long term encoding in which information is encoded, stored, and retrieved. The surgical planning in drug refractory epilepsy (DRE) patients is usually associated with memory deficits. We measured BOLD activation during semantic verbal memory task using auditory stimulus and correlation with clinical parameters in patients (pre and 6 month of post-surgery) and controls. Study revealed anterior temporal lobe resection (ATLR) including removal of medial structures surrounding anterior temporal lobe may develop memory deficits in left TLE patients.
Introduction
The surgical planning in temporal lobe epilepsy (TLE) and extra temporal lobe epilepsy (ETLE) patients is usually associated with memory deficits. The study was aimed to measure BOLD activation during semantic verbal memory task using auditory stimulus and correlation with clinical parameters (age of seizure onset, duration and PGI memory assessment) in cases (pre and 6 month of post-surgery) and controls.Method
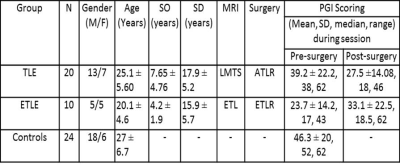
After obtaining the institute ethics approval, 30 consecutive patients with temporal (n=20, left mesial temporal sclerosis) and extra temporal lobe epilepsy (n=7 left frontal lesion; n=2 left parieto-temporal and n=1 posterior occipito-temporal lesion) and 24 healthy controls were recruited in this study (Table 1). Standard diagnostic and exclusion criteria were followed. Auditory cue of a standardized story in Hindi, using Super Lab presentation software was given to the subjects using MR compatible auditory interface system (NordicNeuroLab, Norway). After the story, patients were instructed to speak the answers in this story based questions during fMRI scan.The fMRI sessions were carried out using 1.5T MR scanner (Avanto, Siemens, Germany) using 8 channel head coil. The stimuli were presented using a MR compatible audio visual stimulus system with binocular goggles (Nordic Neuro Lab, Norway). Single-shot echo planar imaging (EPI) sequence was used for the BOLD studies (number of slices: 29, slice thickness 4.5 mm; TR: 2000 ms, TE: 24 ms, FOV: 230 mm, resolution: 64x64 and total number of measurements: 72). Data analysis and group comparisons were carried out using SPM8 (p≤0.001, cluster threshold 10).Results and discussion
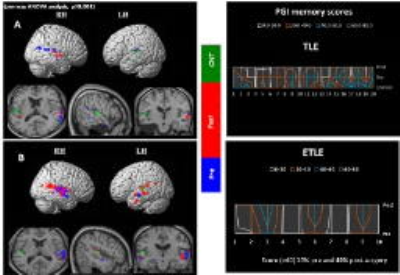
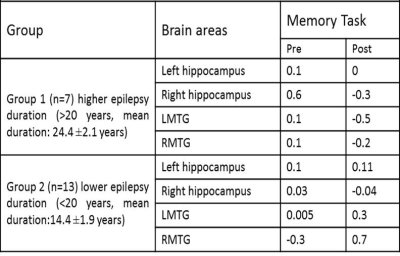
The intra group comparison analysis of BOLD activation in TLE subjects during the memory task, elicited significant BOLD activation in left MTG during pre-surgery than post-surgery. Right MTG along with hippocampal gyrus was hyper active during post-surgery compared to pre-surgery. In ETLE group significant BOLD activation was observed in bilateral MTG along with hippocampal gyrus in pre-surgical than post-surgical session. Clinical memory assessment was abnormal in TLE compared to ETLE patients after 6 months of surgery (Figure 1). Negative correlation was found in LMTG in patients with higher seizure duration. Greater activation in hippocampus and LMTG correlated positively with lower seizure duration (Table 2). The present study revealed changes in mesial temporal lobe memory-encoding activation post-surgery in comparison to pre-surgery in the TLE group. Similar recent studies have been revealed memory dysfunction with longer seizure duration and resection of mesial temporal lobe [1-4]. ETLE group did not elicit significant change in mesial temporal lobe during memory-encoding activation and clinical assessment also revealed improvement post-surgery in comparison to pre-surgery because of preserved memory function during surgery.Conclusion
Anterior temporal lobe resection including removal of medial structures surrounding anterior temporal lobe may cause changes in shape, size, configuration and neuronal networks in temporal lobe region.Acknowledgements
Deparment of Neurology and NMR AIIMSReferences
1. Bonelli, S. et al., 2010. Brain.133, 1186–1199.
2. Powell, H. et al., 2008. JNNP.79, 686–693.
3. Sidhu, M. et al., 2013. Brain.136, 1868-1888.
4. Sidhu MK, et al., 2015. Epilepsy Research. 110, 1-9.
Figures