5567
Effects of smoking cessation treatment on cognition of smokers by using varenicline: a fMRI study1Radiology, Beijing Chao-yang Hospital, Beijing, China, 2School of Biomedical Engineering, Capital Medical University, Beijing, China, 3Clinical Research Center, Beijing Chao-yang Hospital, Beijing, China
Synopsis
This study investigated the working memory-related brain activity to 3 different difficulty level n-back task before and after cessation treatment. Brain activation increased with increasing task difficulty in both control and smoker groups. The brain activation of smokers was higher than that of the control group, but the activation degree of smoking cessation treatment group was significantly lower than that before treatment, which was close to that of the control group. The result demonstrated varenicline’s cure effect on improving cognitive function, which was important to improve the withdrawal symptoms and increase smoking cessation rate.
Abstract
Purpose: Smoking can influence cognition and behavior, including attention1,2, visual information processing3, calculation power and reaction4,5. The effect of smoking in working memory is often the focus of researches. Because nicotine abstinence will induce uncomfortable psychological effects such as lack of concentration, hypomnesia and so on, and many ex-smokers relapsed smoking in order to reduce the withdrawal symptoms. Therefore, how to reduce the withdrawal symptoms and improve the cognitive status during the cessation period is very important to improve smoking cessation rate. In the present analysis, we aimed to measure smokers’ cognitive activation changing patterns during a working memory task (1-back to 3-back), prior to and after smoking cessation treatment by using varenicline, a nicotinic receptor blocker.
Methods: Data were collected using a MAGNETOM Prisma 3T MR scanner with a 64-channel head coil. 23 smokers (18 males and 5 females, mean age 30.7±4.2) and 21 age and gender matched controls were collected. In one fMRI session, subjects needed to finish 3 groups of n-back task. The first group would performance from 1 to 3-back task sequentially. The 1-back task required subjects to keep in memory a series of 20 letters that were being updated constantly. The letters would present one at a time in the middle of a screen while subjects could see it through a mirror on the head coil. They appeared for 2000 msec at intervals of 2000 msec. The subjects were asked to click on a response key to judge if the letter was repeated with the one before. After a shot rest, the subjects would performance 2 and 3-back tasks, which needed them to judge if the letter was repeated with exactly one or two intervening letters before. After a slightly longer rest, the subjects would do other 2 groups of n-back tasks with an upset order of these three level of judging tasks. Smokers were scanned again 1 month after using varenicline. The fMRI scanning parameters were as follows: TR/TE=2000/30 ms; total slices=33; slice thickness=3.5 mm; flip angle=90°; in-plane voxel size=3.5×3.5×3.5 mm3; Measurements=318; total acquisition time=10:42 min. The data were processed using SPM12 which including bias correction, tissue classification, and data registration. Analyses were subsequently performed to compare brain activation between judging and rest intervals (One sample t test, p<0.01).
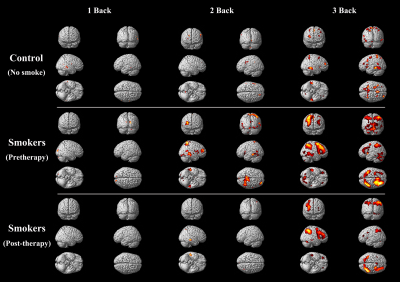
Results: We found that with the increase of the difficulty of judging task, the range of brain activation was also increasing both in control and smoker groups. The brain activation regions of control group were less than smoker groups in each level of cognitive tasks. And the range of brain activation all reduced significantly after cessation treatment than pre-quit status. Especially in areas of cerebellum, superior/inferior parietal lobule, middle/superior frontal gyrus, middle/inferior temporal gyrus (tables1-3,figure1). The accurate judgment rate of smokers after treatment was also obviously improved than before cessation.
Discussion: Previous studies showed that acute nicotine intake can improve cognitive function6. But chronic addiction and nicotine withdrawal could lead to cognitive decline3,7,8. This is also the reason of difficult to quit smoking after dependence founded. As the only cessation medicine without nicotine composition in the world, varenicline has become a research hot spot in recent years9-11. The treatment and placebo group were set up in most studies, and brain activation degree were compared between the two groups after treatment, while they found the range of brain activation has expanded and the time to complete the task accurately was also shortened in treatment group. However, in the absence of non-smoking controls, we can’t accurately judge whether the expansion of the brain activation range equate to cognition improvement. In our study, non-smokers’ brain activation range was the lowest in all groups. This may because controls don’t need to mobilize too many brain regions to complete the working memory task as their normal cognitive function. Smokers had the largest activation areas, which may because their impaired cognition, and more brain areas needed to be actived to complete cognitive tasks. After cessation treatment, less activation of the brain areas which in charge of visuospatial, emotional and memory processing, could complete the same tasks with better cognitive performance, especially in the implementation of high level cognitive tasks (2/3 back). This can prove that varenicline has good improvement effect on cognitive function in smokers.
Conclusions: This study provides novel evidence that the varenicline can improve working memory-related brain activity after 1 month treatment, particularly at high levels of task difficulty. This is important to improve the withdrawal symptoms, increase the rate of smoking cessation and prevent relapse.
Acknowledgements
We thank the World Health Organization Collaborating Centre for Tobacco or Health and the Beijing Institute of Respiratory Medicine for their assistance with the recruitment of smokers and data collection.References
1. Hahn B, Ross TJ, Yang Y, et al. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci, 2007, 27(13): 3477-3489.
2. Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. Eur J Pharmacol, 2000, 393(1-3): 141-146.
3.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron, 2002, 36(3): 539-548.
4. Myers CS, Taylor RC, Moolchan ET, et al. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology, 2008, 33(3): 588-598.
5. Mancuso G, Andres P, Ansseau M, et al. Effects of nicotine administered via a transdermal delivery system on vigilanc: a repeated measure study. Psychopharmacology, 1999, 142(1): 18-23.
6. Sutherland MT, Ross TJ, Shakleya DM, et al. Chronic smoking, but not acute nicotine administration, modulates neural correlates of working memory. Psychopharmacology, 2011, 213(1): 29-42.
7. Kozink RV, Lutz AM, Rose JE, et al. Smoking withdrawal shifts the spatiotemporal dynamics of neurocognition. Addict Biol, 2010, 15(4): 480-490.
8. Sweet LH, Mulligan RC, Finnerty CE, et al. Effects of nicotine withdrawal on verbal working memory and associated brain response. Psychiatry Res, 2010, 183(1): 69-74.
9. Loughead J, Ray R, Wileyto EP, et al.Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010 Apr 15;67(8):715-721.
10.Smith RC, Amiaz R, Si TM, et al.Varenicline Effects on Smoking, Cognition, and Psychiatric Symptoms in Schizophrenia: A Double-Blind Randomized Trial. PLoS One. 2016 Jan 5;11(1):e0143490. doi: 10.1371.
11.Patterson F, Jepson C, Strasser AA, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009 Jan 15;65(2):144-149.
Figures