5566
fMRI-measured brain responses to smoking-related images before and after smoking cessation treatment by varenicline1Radiology, Beijing Chao-yang Hospital, Beijing, China, 2School of Biomedical Engineering, Capital Medical University, Beijing, China, 3Clinical Research Center, Beijing Chao-yang Hospital, Beijing, China
Synopsis
This study investigated the activation of brain regions to visual smoking cues in smokers before and after cessation treatment using an event-related design fMRI. The result demonstrated that smokers will have good tolerance to smoking-related stimuli after using varenicline. This may explain why the curative effect of varenicline is better than NRT which has been related to an increased the brain response. The results provide further evidence for medication choice of clinicians for smoking cessation.
Abstract
Purpose: Smoking is an addictive disease that can easily relapse. Even if the physiological addiction has been lifted, smoking-related cues may induce psychological addiction and thus cause relapse. Smokers’ brain reactivity to smoking-related cues may play an important role in smoking addiction and the maintenance of smoking cessation status. Several previous studies have examined smokers’ brain response to smoking-related images in different status, such as in nicotine abstinence status and in resting status after cessation1,2. However, little is known about the changes in brain responses caused by smoking cessation treatment. Therefore we performed the current study aiming to evaluate the differences in patterns of spontaneous brain activity before and after smoking cessation treatment by using varenicline, a nicotinic receptor blocker.
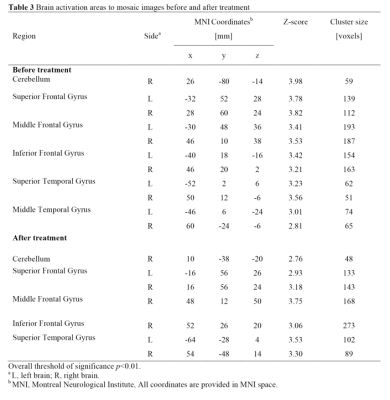
Methods: Data were collected using a MAGNETOM Prisma 3T MR scanner with a 64-channel head coil from a total of 23 smokers (18 males and 5 females). They aged mean 23-40 years, with the mean (standard deviation, SD) age as 30.7 (4.2) years, when the study was performed. We used an event-related design functional magnetic resonance imaging (fMRI) to quantify the responses of subjects to three different types of images, namely smoking-related images, neutral images and mosaic images(Figure 1). The latter two were used as controls to reveal the response directly related to smoking cues. The scan parameters were as follow: TR/TE=3000/30 ms; total slices=33; slice thickness=3.5 mm; flip angle=90°; in-plane voxel size=3.5×3.5×3.5 mm3; Measurements=201; total acquisition time=10:11 min. Images were displayed in random order and subjects were asked to click on a response key when they saw the images. All subjects were scanned immediately before and 1 month after varenicline treatment. The data were processed using SPM12 which included bias correction, tissue classification, and data registration. One-sample T-test was performed to compare brain activation stimulated by different types of images with no task status separately, before and after cessation treatment (p<0.01 was considered significant).
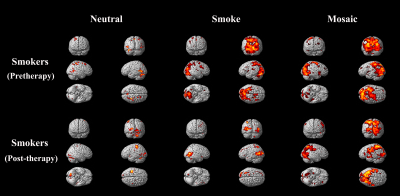
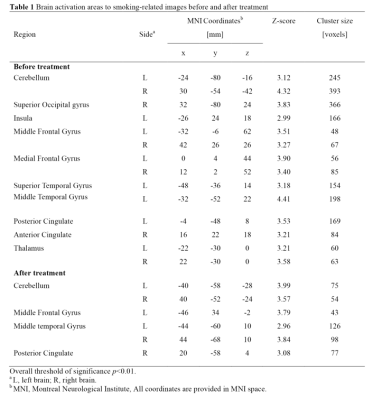
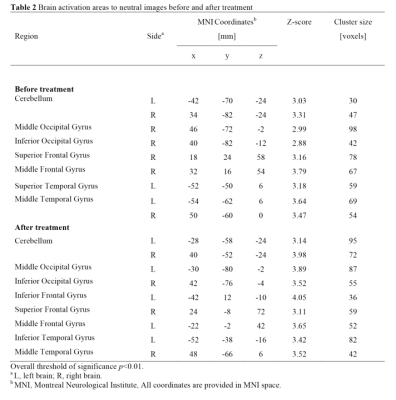
Results: We found that neutral images activated the least brain area, while the other two kinds of images both led to obvious activation regions. However, only the activation area of smoking-related images was significantly reduced after smoking cessation, especially in areas of grey matter of cerebellum, superior occipital gyrus, insula, superior temporal gyrus, posterior cingulate, anterior cingulate and thalamus (Table 1-3; Figure 2).
Discussion: Previous findings suggest that brain reactivity of smokers to smoking-related images was increased in emotional processing regions which including the frontal and anterior cingulate cortex3,4, visuospatial processing regions which including parietal and occipital cortices5,6. This is consistent with our findings. Previous research has found that brain activation stimulated by smoking-related images increased obviously in abstinence status induced by nicotine replacement therapy (NRT)2. Prefrontal, anterior and posterior cingulate, temporal, parietal cortex, and caudate nucleus were all more pronounced activation than pre-quit state. Those regions are known to be responsible for the action planning, habit learning, and craving and activation enhancement of these areas could increase relapse vulnerability7-12. Such impact of NRT treatment is reasonable because NRT is a kind of harm reduction treatment, but not fundamentally addiction treatment. On the other hands, as a nicotinic receptor blocker, varenicline can significantly reduce smokers’ euphoric feeling from smoking, therefore facilitate smoking cessation. Our fMRI results also show that brain activity in areas in charge of habit learning, action planning and craving from smokers treated with varenicline to smoking cues was reduced, in contrast to the impact of NRT treatment. Such reduction is very important from the maintenance of smoking cessation status, may explain why smoking cessation efficacy of varenicline is significantly higher than that of NRT13-16. We used mosaic images as another control to compare brain’s response to smoking and non-smoking related cues before and after treatment. The brain activation by mosaic images is obvious than neutral images and that might be related to the unclarity and meaningless of the images which stimulate the brain to explore and analyze. But this activation did not change significantly before and after smoking cessation, that was same as neutral image group.
Conclusions: These data suggest that compare to control groups, smoking-related images can obviously activate brain areas involved in action planning, habit learning, and craving processing. Using varenicline can reduce smokers’ response to smoking stimuli, which relate to its pharmacological mechanism and contribute to better therapeutic effect of smoking cessation.
Acknowledgements
We thank the World Health Organization Collaborating Centre for Tobacco or Health for their assistance with the recruitment of smokers.References
1. Chao W, Zhujing S, Peiyu H, et al. Altered spontaneous activity of posterior cingulate cortex and superior temporal gyrus are associated with a smoking cessation treatment outcome using varenicline revealedby regional homogeneity. Brain Imaging Behav. 2017;11(3):611-618.
2. Janes AC, Frederick Bd, Richardt S, et al. Brain fMRI responses to smoking-related images prior to and during extended smoking abstinence. Exp Clin Psychopharmacol. 2009;17(6):365-373.
3. Fichtenholtz HM, Dean HL, Dillon DG, et al. Emotion-attention network interactions during a visual oddball task. Brain Research Cognitive Brain Research. 2004;20(1):67–80.
4. Keightley ML, Winocur G, Graham SJ, et al. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41(5):585–596.
5. McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33(9):2148–2157.
6. Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30(4):810–820.
7. Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. The Journal of Neuroscience. 2006;26(13):3584–3588.
8. Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. The American Journal of Psychiatry. 2000;157(11):1789–1798.
9. Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006; 31(3):644–650.
10. See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berlin). 2007;194(3):321–331.
11. Smolka MN, Buhler M, Klein S, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berlin). 2006;184(3-4):577–588.
12. Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience. 2007;145(1):288–302. 13. Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of varenicline versus nicotine replacement therapy for smoking cessation in the "real world". BMC Public Health. 2014;14:1163.
14. Kotz D, Brown J, West R. Effectiveness of varenicline versus nicotine replacement therapy for smoking cessation with minimal professional support: evidence from an English population study. Psychopharmacology (Berl). 2014;231(1):37-42.
15. Mills EJ, Wu P, Lockhart I, et al. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: A systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588-597.
16. Hsueh KC, Hsueh SC, Chou MY, et al. Varenicline versus transdermal nicotine patch: a 3-year follow-up in a smoking cessation clinic in Taiwan. Psychopharmacology (Berl). 2014;231(14):2819-2823.
Figures