5564
The Effect of HIV and Aging on Functional Connectivity, Connectome Similarity, and Structural changes1Electrical and Computer Engineering, University of Rochester, Rochester, NY, United States, 2Dept of Biostatistics and Computational Biology, University of Rochester, Rochester, NY, United States, 3Department of Imaging Sciences, University of Rochester, Rochester, NY, United States, 4Department of Neurology, University of Rochester, Rochester, NY, United States
Synopsis
We investigated the effect of HIV-infection and aging using resting-state fMRI and T1-weighted structural images. First, we assessed the inter-network functional connectivity and identified 8 resting-state networks associate with normal aging. Second, we constructed a functional connectome similarity as a global measure of neural connectivity pattern to assess the effect of age and HIV infection. Lastly, we measured cortical thickness and brain volumetrics. We found a significant effect of aging but not a definite additive or synergistic effect of HIV infection in all three analyses.
Introduction
Combination antiretroviral therapy (cART) has changed the natural history of HIV infection; however, the chronic and persistent immune activation and possibly the effect of cART itself may accelerate biological aging1-5. However, whether there is an interaction of HIV-infection and aging on functional connectivity(FC) is still debatable3. In our study, we approached this question by using inter-network FC and similarity analysis to examine how neural connectivity pattern changes in HIV-infection and aging. Structural assessment was also performed as a standard approach in measuring brain aging.Methods
MRI data was acquired on a 3T Siemens MAGNETOM Trio MRI scanner equipped with a 32-channel head coil. A T1-weighted 3D MPRAGE(TR/TI/TE= 2530/1100/3.44 ms, voxel size=1x1x1mm3, flip angle(FA)=78°, bandwidth=190 Hz/pixel) was used for anatomic images. Resting-state functional MRI was acquired using a gradient echo-planar imaging (EPI) sequence (TR/TE=2000/30 ms, FA=90°, voxel size=4x4x4mm3; matrix=64x64, 30 axial slices, volumes=150).
Standard rs-fMRI pre-processing steps were performed using DPARSF6 and SPM12, including slice-timing correction, head motion correction, co-registration, normalization, spatial smoothing with Gaussian kernel (FWHM=4mm), detrend, and band-pass filtering(0.01-0.08Hz). Nuisance regression includes: 6 head motion parameters, white matter and cerebrospinal fluid signal. Group ICA was then performed on HIV-negative to determine resting-state networks (RSN) with GIFT(http://mialab.mrn.org/software/gift/index.html). Time courses of each individual for each RSN were used to create the FC matrix (connectome) by correlating all possible pairs of time courses of RSNs. Age ≤ 35 and ≥40 was used as cutoff age to separate subjects into young and old groups, respectively.
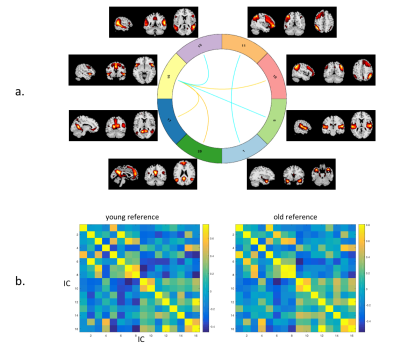
Firstly, all the functional connectomes were compared using multiple regression and two sample t-test. Secondly, within the HIV-uninfected, the Fisher z-transformed connectomes were averaged to create two connectome references, one for older and one for younger participants. For HIV-infected subjects, each connectome were vectorised, and correlated with the young and old connectome references(Figure. 2b) separately. Connectome similarity was defined as the Fisher z-transformed score of the Pearson-correlation of the connectome to the reference connectome. Thirdly, cortical thickness and brain volumes were extracted using Freesurfer. Volume of the each regions of interest (ROI) was normalized by individual’s total intracranial volume, including: caudate, putamen, pallidum, hippocampus, amygdala, lateral ventricle on both hemispheres. The multiple regression was performed on cortical thickness and brain volumes to test the effect of HIV and Age and the interaction effect, with both HIV and Age were used as categorical factors.
Results
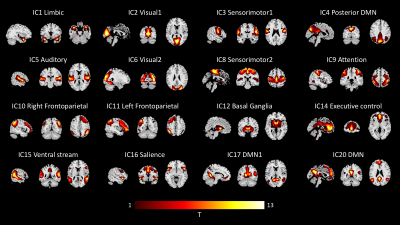
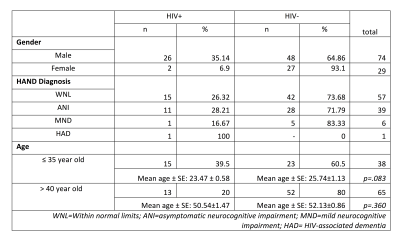
Twenty-eight HIV-infected subjects and seventy-five age-matched HIV-uninfected were included in this study. Within each young/old group, no group difference was significant compare HIV-infected and HIV-uninfected(Table 1.).Twenty independent components (IC) were extracted, while 4 of them are physiological and head motion noise. We visually identified 16 ICs as RSNs and used for further analysis(Figure.1).
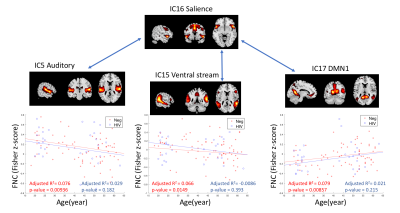
In the first inter-network FC analysis, we did not find an HIVxAge interaction effect. A significant age effect in HIV-uninfected individuals was found in six pairs of RSN (p<0.05, FDR corrected) (Figure. 2a). The correlation between each pair of RSNs was fed into a regression model against age as a continuous variable. Three pairs of inter-network FC showed significant linear relationship with age (adjusted R2= 0.066-0.079, p<0.05) in the HIV-uninfected group, but such a linear relationship was not observed in the HIV-infected group, see Figure. 3.
In similarity analyses, the HIV-infected old group showed significantly higher similarity to the old reference than to the young reference (p<0.01) suggesting that the older HIV infected group behaves similarly than older age match controls. On the other hand, the HIV-infected young individuals showed no significant similarities with neither the young or older reference control group, suggesting a possible effect of HIV infection.
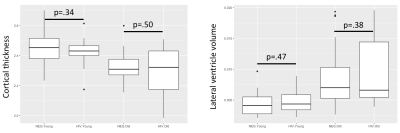
In the multiple regression analysis of cortical thickness and volumetric, we did not find any HIVxAge interaction effect, while we did see that age had a significant main effect on cortical thickness (p=1.2e-06), lateral ventricle (left:p=1.3e-04, right:p=7.04e-05), thalamus (left: p=.032, right: p=.0099), caudate (left: p=.031, right: p=.0094), putamen (left: p=.0087, right: p=1.93e-04), 3rd ventricle(p=6.33e-04), accumbens (left: p=.0054). Figure 4. shows two examples of the areas assessed.
Discussion
In the first analysis, five out of six pairs of internet-work FC changes involved the salience networks. This is expected since salience network is needed to switch between the central executive network and the DMN7, and is sensitive to normal aging8. The similarity analyses suggest that the interaction between HIV and aging is most visible in the younger group, which shows FC in between the two reference HIV-uninfected control groups. None of the structural analyses suggested an HIV-aging interaction.Conclusion
The effect of HIV on normal aging does not appear to follow a linear relationship as indicate by a larger effect on similarities in the younger HIV infected group than in older HIV infected group.Acknowledgements
No acknowledgement found.References
1. Watson, C., et al., White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J Neurovirol, 2017. 23(3): p. 422-429.
2. Kuhn, T., et al., The effects of HIV and aging on subcortical shape alterations: A 3D morphometric study. Hum Brain Mapp, 2017. 38(2): p. 1025-1037.
3. Hakkers, C.S., et al., Review of functional MRI in HIV: effects of aging and medication. J Neurovirol, 2017. 23(1): p. 20-32.
4. Cole, J.H., et al., Increased brain-predicted aging in treated HIV disease. Neurology, 2017. 88(14): p. 1349-1357.
5. Seider, T.R., et al., Age exacerbates HIV-associated white matter abnormalities. J Neurovirol, 2016. 22(2): p. 201-12.
6. Chao-Gan, Y. and Z. Yu-Feng, DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci, 2010. 4: p. 13.
7. He, X., et al., Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp, 2014. 35(7): p. 3446-64.
8. La Corte, V., et al., Cognitive Decline and Reorganization of Functional Connectivity in Healthy Aging: The Pivotal Role of the Salience Network in the Prediction of Age and Cognitive Performances. Frontiers in Aging Neuroscience, 2016. 8.
Figures