5563
Altered functional connectivity between cerebral and cerebellar resting-state networks in autism spectrum disorderYan Wang1, Wenjing Zhang1, Zheng Wang2,3,4, Jieke Liu1, John A. Sweeney1,5, Stormi P. White6, Su Lui1, and Matthew W. Mosconi2,3,4
1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2Schiefelbusch Institute for Life Span Studies, University of Kansas, Lawrence, KS, United States, 3Clinical Child Psychology Program, University of Kansas, Lawrence, KS, United States, 4Kansas Center for Autism Research and Training (K-CART), University of Kansas, Lawrence, KS, United States, 5Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 6Center for Autism and Developmental Disabilities, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
By examining the correlation between motor data and brain resting-state functional imaging and identifying the functional connectivity alteration in ASD using a seed-driven approach, individuals with ASD demonstrate altered pattern of motor activation and diffusely decreased FC within frontal-subcortical-cerebellar circuit and within cerebellar network, which may represent the underlying neurobiological mechanisms of motor dysfunction and further delayed acquisition of gestures important for socialization and communication.
INTRODUCTION
Motor impairments have been consistently documented in autism spectrum disorder (ASD). Recent evidence suggests that the onset of motor dysfunctions may precede the emergence of social and communication deficits in the first year of life in ASD1. Despite that the neurophysiological substrates of motor skills have been well-characterized via animal and human lesion studies, there are still far less imaging studies about the ASD motor impairment than the core social communication and cognitive features. Most of the motor functional connectivity (FC) imaging researches are task-based 2–4.However, task-based FC complicates discerning whether observed connectivity is driven by task-induced activity or intrinsic task-unrelated ongoing fluctuations5 and it’s susceptible to subject compliance and task performance across windows6. Therefore, our purpose is to examine the correlation between motor data and brain resting-state functional imaging and identify the functional connectivity alteration in ASD using a seed-driven approach.METHODS
Forty-three individuals(24 ASD) were recruited in the study, aging from 10 to 33 years ( ASD,19.09±5.90;control,23.31±4.11). All patients met diagnostic criteria for autism according to the Autism Diagnostic Inventory-Revised (ADI-R)/Autism Diagnostic Observation Schedule (ADOS) and DSM-V criteria. All participants underwent MRI scanning on a GE 3T system with an 8-channel head coil. An echo planar sequence was used to acquire resting-state fMRI sensitized to changes in BOLD signal levels (TR/TE=1500/25msec, flip angle=60°, FOV 220x114.2x220 mm, slice thickness=3.4mm,1mm gap, 33 axial slices, 240 volumes in each run). High-resolution structural scans were acquired using MPRAGE protocol to facilitate registration to standard space (TR/TE= 8.1/3.373 ms; FOV 256x204x160 mm; 1-mm isotropic voxels, 160 sagittal slices). Ten individuals with ASD and 11 control participants completed both precision gripping behavioral and rs-fMRI imaging tests, and the standard deviation (mean.SD.60) of the sustained force was examined to quantify individuals’ motor accuracy and within-participant mean.SD.60 was calculated as individuals’ behavioral measure. Imaging preprocessing was performed with Data Processing Assistant for Resting-State fMRI Basic Edition (DPARSF_3.0; http://rfmri.org/DPARSF). Statistical analytic processes were applied in REST software (Resting-State fMRI Data Analysis Toolkit V1.8; http://restfmri.net/forum/rest). First, the voxel-wise correlation between ALFF and motor data (mean.SD.60)was calculated within patient and control groups separately. Then the regions which significantly correlated with motor performance were selected as ROIs for the further FC analysis. In all image analyses, an approach which utilizes Monte Carlo simulations, namely AlphSim calculation, was used to correct for multiple comparisons. The probability of a false-positive detection for analyses was set to p<0.05 using a minimum cluster size of 5 contiguous voxels significantly different at a nominal threshold of p<0.05 (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf).Brain-behavior relationships were assessed by regressing functional connectivity measures with social deficit severity (ADOS) and repetitive behavior (RBS-R total).RESULTS
We identified brain regions which showed mainly significant positive correlation with motor data as ROIs in ASD and control group separately,primarily including posterolateral cerebellum, superior temporal, left angular and right supramarginal gyrus in controls, and left temporal, left precuneus, right precentral and left postcentral gyrus in ASD. Whole-brain analysis for regions of interest showed different patterns of connectivity in the patient and control groups. Relative to control participants, individuals with ASD showed a complex pattern of connectivity in the cerebral-cerebellar network, primarily lower connectivity in seed left angular、posterolateral cerebellum with right superior frontal gyrus but higher connectivity with superior occipital gyrus. Lower connectivity was also identified within cerebellar network, mainly between seed posterolateral cerebellum and right cerebellum crus II. Additionally,for seed left heschl gyrus and precuneus,there was higher connectivity with superior parietal gyrus but lower connectivity with right cerebellum crus II. The FC between left precuneus and right superior parietal gyrus showed negative correlation with RBS-R total scores in the ASD group (P=0.024, q[FDR]=0.048).DISCUSSION
Individuals with ASD showed primarily greater correlation with motor data in primary motor and somatosensory cortex(precentral and postcentral gyrus)while the control group showed mainly greater correlation in posterolateral cerebellum. The inter-group differences in pattern of cerebral and cerebellar motor activation may represent the underlying neurobiological mechanisms of motor dysfunction in individuals with autism. Additionally, the ASD group which showed diffusely decreased connectivity within frontal-subcortical-cerebellar circuit and within cerebellar network may reflect poor coordination within the circuit necessary for motor control and learning. The findings might explain impairments in motor development in autism, as well as abnormal and delayed acquisition of gestures important for socialization and communication.CONCLUSION
This study demonstrates altered pattern of motor activation and diffusely decreased FC within frontal-subcortical-cerebellar circuit and within cerebellar network in individuals with autism, which may represent the underlying neurobiological mechanisms of motor dysfunction and further delayed acquisition of gestures important for socialization and communication.Acknowledgements
We acknowledge Ms. Shannon E. Kelly for her assistance in organizing behavioral, clinical diagnostic and neuroimaging data.Grant: Study funded by NIMH K23 Research Career Development Award (MH092696), NIMH R01 Research Project Grant Program (MH 112734), Once Upon a Time Foundation Award, the Kansas Center for Autism Research and Training (K-CART) Research Investment Council Strategic Initiative Grant to Dr. Mosconi, and the NICHD U54 Kansas Intellectual and Developmental Disabilities Research Center Award (U54HD090216).References
- Baranek, G. T. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. J. Autism Dev. Disord. 29, 213–224 (1999).
- Allen, G. & Courchesne, E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: An fMRI study of autism. Am. J. Psychiatry 160, 262–273 (2003).
- Allen, G., Müller, R. A. & Courchesne, E. Cerebellar function in autism: Functional magnetic resonance image activation during a simple motor task. Biol. Psychiatry 56, 269–278 (2004).
- Mostofsky, S. H. et al. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 132, 2413–2425 (2009).
- Sadaghiani, S. & Kleinschmidt, A. Functional interactions between intrinsic brain activity and behavior. Neuroimage 80, 379–386 (2013).
- Gonzalez-Castillo, J. & Bandettini, P. A. Task-based dynamic functional connectivity: Recent findings and open questions. NeuroImage (2017). doi:10.1016/j.neuroimage.2017.08.006
Figures
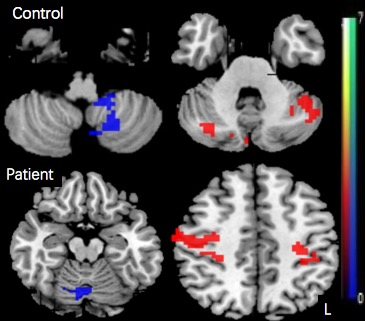
Figure 1.The brain regions which significantly correlated
with motor data. Warm color (positive value) indicated regions which showed
positive correlation with motor data,while cool color
(negative value) indicated negative correlation with motor data.
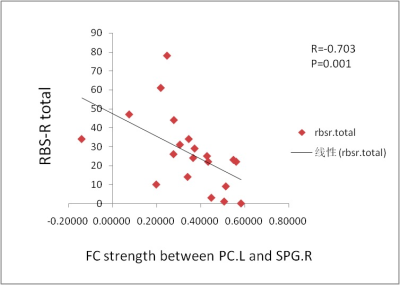
Figure 2. The scatterplot shows the relationship between the PC.L and SPG.R connectivity (average z scores) and restricted and repetitive behavior measured by the RBS-R total scores (Spearman r =-0. 703, P = .001). FC, functional connectivity; PC.L, left precuneus; SPG.R, right superior parietal gyrus;RBS-R total, repetitive behaviors scale-revised total score.