5559
Disrupted resting-state functional connectivity and low-frequency fluctuation in chronic pain1Seoul National University, Seoul, Korea, Democratic People's Republic of
Synopsis
By applying dynamic functional connectivity and low-frequency fluctuation analysis of resting-state fMRI data, the present study investigated a disrupted intrinsic resting-state network in chronic pain patients.
Introduction
Though various human brain imaging studies have revealed specific brain changes in chronic pain, those are mostly focused on brain responses to painful and non-painful stimulation.1,2,3 Therefore, the resting-state network is still understudied in chronic pain with few studies showing incompatible results.4 Without any experimental stimulation, the human brain produces low-frequency fluctuations and these feature intrinsic resting-state network. Here, the present study was conducted to identify such features by examining the resting-state functional MRI (fMRI) in chronic pain patients.Methods
All fMRI data were acquired with a 3.0T MR scanner (Siemens Magnetom TrioTim) in age-, sex-, and education-matched 20 healthy controls and 12 fibromyalgia patients. Resting-state echo-planar imaging used the following parameters: echo time = 30 ms; repetition time = 3500 ms; flip angle = 90; 35 slices; 116 contiguous functional volumes; field of view = 240 mm; voxel size = 1.9 x 1.9 x 3.5 mm. T1-weighted images for coregistration were acquired under the following parameters: echo time = 1.89 ms; repetition time 1670 ms; flip angle = 9; 208 slices; matrix = 256 x 256; field of view = 250 mm. After pre-processing (realignment, coregistration, normalization, detrending, band-pass filtering) of the resting-state BOLD images using SPM8 and Data Processing Assistant for Resting-state fMRI (DPARSF), for each subject, we used a sliding window approach to estimate the dynamic functional connectivity with seed of rACC/mPFC (x = - 6, y = 54, z = - 3; 3 mm radius sphere of MNI coordinate) through Dynamic brain connectome analysis toolbox. It allows to estimate the dynamic functional connectivity over the low-frequency band of interest (0.027-0.073 Hz).5,6 For calculating the low-frequency fluctuations, we calculated the amplitude of low-frequency fluctuation (ALFF) and fractional ALFF within each voxel by using DPABI: a toolbox for Data Processing and Analysis for Brain Imaging. Significant group differences between the fibromyalgia patients and healthy controls were calculated using 2 sample t-tests (p < 0.05).Results
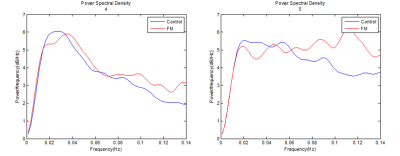
A group comparison of dynamic functional connectivity (seed: rACC/mPFC based on MNI coordinate) showed that the connectivity between rACC/mPFC - precuneus, a known part of the default mode network (DMN), was significantly increased in fibromyalgia patients compared to healthy controls. As for the comparison of infra-slow oscillation power (ie, ALFF and fALFF) between 0.027-0.073 Hz displayed significantly reduction in fibromyalgia patients in rACC and mPFC.Discussion
Consistent with neuroimaging studies in chronic pain, the present study found that DMN functional dynamics were significantly altered in fibromyalgia patients at rest. By applying the concept of dynamic functional connectivity using regularized covariance matrix, though in resting-state, the BOLD fluctuation was calculated in chronic pain patients for the first time. Further, the disruption in the resting-state network was confirmed by increased ALFF and fALFF in the rACC and mPFC which deal with descending pain modulation. Therefore, the present study investigated the rapid nature of resting state network in chronic pain patients through connectivity and frequency fluctuation analyses.Conclusion
Fibromyalgia patients demonstrated increased dynamic functional connectivity between rACC/mPFC and precuneus and reduced infra-slow oscillation power at rest, which indicates their disrupted resting-state intrinsic functional network in chronic pain.Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1904984).References
1. Lim M, Roosink M, Kim JS, et al. Disinhibition of the primary somatosensory cortex in patients with fibromyalgia. Pain. 2015 Apr;156(4):666-74.
2. Choi W, Lim M, Kim JS, et al. Habituation deficit of auditory N100m in patients with fibromyalgia. Eur J Pain. 2016 Nov;20(10):1634-1643.
3. Lim M, Roosink M, Kim JS, et al. Augmented Pain Processing in Primary and Secondary Somatosensory Cortex in Fibromyalgia: A Magnetoencephalography Study Using Intra-Epidermal Electrical Stimulation. PLoS ONE. 2016 Mar 18;11(3):e0151776
4. Kim JY, Kim SH, Seo J, et al. Increased power spectral density in resting-state pain related brain networks in fibromyalgia. Pain. 2013 Sep;154(9):1792-7
5. Yan CG, Wang XD, Zuo XN, et al. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016 Jul;14(3):339-51.
6. Xuhong Liao, Lin Yuan, Tengda Zhao, et al. Spontaneous functional network dynamics and associated structural substrates in the human brain. Front Hum Neurosci. 2015; 9: 478.