5558
Application of fMRI with seed-based analysis for assessment of functional connectivity in patients with fragile X syndrome1Novosibirsk State University, Novosibirsk, Russian Federation, 2Institute International Tomography Center SB RAS, Novosibirsk, Russian Federation, 3Medical Сenter «Avicenna», group of companies «Mother and Child», Novosibirsk, Russian Federation, 4Institute of Molecular and Cellular Biology SB RAS, Novosibirsk, Russian Federation, 5Lavrentyev Institute of Hydrodynamics SB RA, Novosibirsk, Russian Federation
Synopsis
The purpose of the study was to find neurobiological correlates of cognitive
disability in patients with fragile X syndrome using resting state fMRI and
seed-based correlation analysis.
Synopsis
The study aimed to find neurobiological correlates of cognitive disability in patients with fragile X syndrome using resting state fMRI and seed-based correlation analysis. Resting state fMRI study (1.5T Philips Achieva) involved two groups of participants: 17 patients with FraX and 8 healthy volunteers. Seed-based FC maps were generated using DPARSFA; group comparison was performed using SPM8. The fMRI study revealed that patients in comparison with controls showed negative functional connectivity between inferior temporal gyri and middle parts of cingulum cortex, occipital and superior temporal gyri, Brodmann area 10 in cooperation with inferior parietal lobe (p-value < 0.0001, unc.).Purpose
Fragile X syndrome (FXS) is one of the most common causes of hereditary mental retardation presenting important social and medical problem [1]. Due to absence of diagnostically significant neurobiological correlates of mental retardation reflecting functional connectivity in these patients. Thus the aim of the study is to compare functional connectivity in regions of interest between patients with fragile X syndrome and healthy control using seed-based analysis of fMRI data.Materials and methods
During the last decade, researchers have been discovering structural [2] and functional brain abnormalities [3] associated with mental retardation among Fragile X patients. However, considering cognitive impairments, extrapyramidal signs, hypermobility of the patients, resting-state fMRI is a method of choice [4]. There are two main methods of analysis of functional connectivity in the brain: Independent component analysis (ICA) and Seed-based analysis (SBA). In the last case, signal from a certain voxel known as the seed or ROI is used to calculate correlations with signals of other voxels of the brain [5]. In our study fMRI was performed using 1.5T Philips Achieva scanner. Resting state fMRI study involved two groups of patients: 17 children with confirmed fragile X (FraX) syndrome and 8 healthy volunteers. Seed-based functional connectivity maps were generated using DPARSFA, group comparison was performed using SPM. DTI data was analyzed for modeling and initial ROI selection.ROIs selection & modeling
In this study, we selected white matter tracts that have been changed according to previous DTI analysis observed performed in our laboratory: cingulum, inferior longitudinal and uncinate fasciculi. We distinguished three main models: 1. FC between inferior temporal gyri and middle parts of cingulum cortex (further called as model 1). 2. FC between occipital and superior temporal gyri (further called as model 2) and 3.FC between Brodmann area 10 and inferior parietal lobe.Results
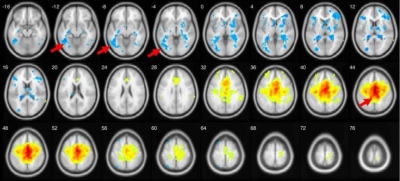
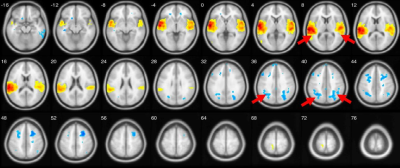
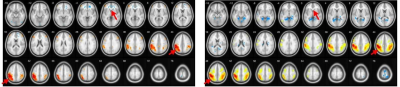
New study using resting state fMRI and seed-based correlation analysis revealed that patients with fragile X syndrome in comparison with controls showed negative functional connectivity between left and right inferior temporal gyrus and middle parts of cingulum cortex (Median Cingulate Region of AAL atlas, both left and right), p-value < 0.0001 uncorrected. Example data shown in Figure1.Only FraX group showed negative functional connectivity between superior temporal gyri and both superior and middle occipital gyri (both ipsi- and contralateral), as visible on left and right superior temporal gyri functional connectivity maps thresholded at p<0.0001, uncorrected. Generalized result is shown in Figure 2.Control group demonstrates strong FC of Brodmann area 10 with inferior parietal gyri compared with FraX. Generalized result is shown in Figure 3.Discussion
Inferior temporal gyrus is anatomical and functional component of ventral visual system providing recognition of face and three-dimensional objects [6]. Thus data from Fig.1 may reflect difficulties in visual-spatial orientation of the patients as well as impaired semantic memory [7]. Superior temporal sulcus and occipital gyrus play role in face recognition important for successful social interaction [8,9]. Data at the Fig.2 may represent neural correlates of impaired social and emotional interaction reflecting ASD symptoms among the patients [10]. Inadequate prefrontal cortex function described in Fig. 3 may explain visible impairments of higher cognitive function, verbal and memory disturbances [11].Conclusion
Seed-based analysis of fMRI data revealed some patterns of functional connectivity for patients with fragile X syndrome in comparison with control subjects. Weaker connections of inferior temporal gyrus and particularly within occipital may be a neural correlate of impaired social and emotional interaction with society among the patients.Acknowledgements
The work was supported by the FASO Russia (project № 0333-2016-0003) regarding theoretical part, the Russian Science Foundation (the project #15-15-10001 regarding genetic analysis and the project #17-11-01156 in part of the work on magnetic resonance imaging).References
[1] Galanina E M, Tulupov A A, Lemskaya N A, Korostyshevskaya A M, Maksimova Y V, Shorina A R, Savelov A A, Sergeeva I G, Isanova E R, Grishchenko I V and Yudkin D V 2017 A Female Patient with FMR1 Premutation and Mosaic X Chromosome Aneuploidy and Two Sons with Intellectual Disability. Mol. Syndromol. 8 110–4
[2] Hallahan B P, Craig M C, Toal F, Daly E M, Moore C J, Ambikapathy A, Robertson D, Murphy K C and Murphy D G M 2011 In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study Neuroimage 54 16–24
[3] Kim S-Y, Hashimoto R, Tassone F, Simon T J and Rivera S M 2013 Altered neural activity of magnitude estimation processing in adults with the fragile X premutation J. Psychiatr. Res. 47 1909–16
[4] Isanova E, Petrovskiy E, Savelov A, Yudkin D and Tulupov A 2017 Resting-state fMRI study of patients with fragile X syndrome J. Phys. Conf. Ser. 886 12007
[5] Van Dijk K R A, Hedden T, Venkataraman A, Evans K C, Lazar S W and Buckner R L 2010 Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization J. Neurophysiol. 103 297–321
[6] Wyss R, König P and Verschure P F M J 2006 A model of the ventral visual system based on temporal stability and local memory PLoS Biol. 4 e120
[7] Chan D, Fox N C, Scahill R I, Crum W R, Whitwell J L, Leschziner G, Rossor A M, Stevens J M, Cipolotti L and Rossor M N 2001 Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease Ann. Neurol. 49 433–42
[8] Leslie K R, Johnson-Frey S H and Grafton S T 2004 Functional imaging of face and hand imitation: towards a motor theory of empathy Neuroimage 21 601–7
[9] Pelphrey K A, Viola R J and McCarthy G 2004 When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus Psychol. Sci. 15 598–603
[10] Lord C, Risi S, Lambrecht L, Cook E H, Leventhal B L, DiLavore P C, Pickles A and Rutter M 2000 The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism J. Autism Dev. Disord. 30 205–23
[11] Koechlin E and Hyafil A 2007 Anterior prefrontal function and the limits of human decisionmaking Science (80-.). 318 594–8
Figures