5554
DEFAULT MODE NETWORK MODIFICATIONS IN FABRY DISEASESirio Cocozza1, Giuseppe Pontillo1, Mario Quarantelli2, Francesco Saccà3, Eleonora Riccio3, Teresa Costabile3, Gaia Olivo4, Vincenzo Brescia Morra3, Antonio Pisani3, Enrico Tedeschi1, and Arturo Brunetti1
1Department of Advanced Biomedical Sciences, University of Naples "Federico II", Naples, Italy, 2Institute of Biostructure and Bioimaging, National Research Council, Naples, Italy, 3University of Naples "Federico II", Naples, Italy, 4Department of Neuroscience, Uppsala University, Uppsala, Sweden
Synopsis
We performed a Resting-State fMRI study to evaluate the integrity of the Default Mode Network (DMN) in Fabry Disease (FD), a condition in which neuropsychological symptoms are common and alterations of the functional connectivity (FC) have been recently reported. Compared to healthy controls FD patients showed clusters of increased FC involving different DMN hubs as well as the middle temporal gyri and the right cerebellum, with a significant correlation with the Corsi span test results (P=0.0001). Our results confirm the current view of a cerebral involvement in FD patients related to significant and diffuse functional changes.
Introduction
Fabry disease (FD) is a rare X-linked metabolic disorder caused by a deficiency in the lysosomal enzyme α -galactosidase A (α-GLA) 1. Neuropsychological symptoms are common in FD, in particular depression, reported to be clinically significant in up to 50% of the patients 2. Furthermore, existing data suggest an impairment in executive functioning, information processing speed and attention , although with a preservation of global cognitive functioning 3. Abnormalities of the Default Mode Network (DMN) have been demonstrated in several disorders (e.g. Alzheimer’s disease 4, Parkinson's disease 5, Huntington's disease 6 and epilepsy 7). However, to the best of our knowledge no studies have been performed to evaluate the integrity of the DMN in FD, a condition in which alterations of the functional connectivity (FC) have been recently reported 8. Therefore, we aimed to perform an RS-fMRI study in FD to evaluate possible alterations of this network and correlations with neuropsychological scores in FD patients, to help understand the mechanisms underlying the cognitive dysfunction in this condition.Methods
Thirty-two FD patients with genetically confirmed diagnosis of classical FD (Males=12, mean age 43.3±12.2) were enrolled, along with thirty-five healthy controls (HC) of comparable age and sex (Males=14, mean age 42.1±14.5). MRI data were acquired on a 3 Tesla MR scanner (Trio, Siemens Medical Systems, Erlangen, Germany). Functional data were processed using the functional connectivity toolbox (CONN, v. 16.a, http://www.nitrc.org/projects/conn), which contains libraries for fMRI analysis based on the Statistical Parametric Mapping (SPM8) software package (http://www.fil.ion.ucl.ac.uk/spm). Briefly, pre-processing steps included the removal of the first five time points, to allow for instability of the initial MRI signal, leaving 195 time points, followed by the motion and slice timing correction, the temporal despiking by means of an hyperbolic tangent squashing function to limit outlier values, band-pass filtering (0.008 Hz<f<0.09 Hz), and spatial smoothing (using a 6-mm Full-Width at Half Maximum Gaussian kernel). Studies with a mean relative root-mean-square (RMS) of the translation parameters at each time point of 0.15 or higher (according to 9), or with more than 1.5mm displacement along or 1.5 degrees rotation around any of the three main axes, were discarded from the analysis. For each subject, BOLD signal time course was calculated separately for each of the main hubs of the DMN, namely the posterior cingulate and the ventral portion of the precuneus (PCC), the medial prefrontal cortex (MPFC), and the right (RPL) and left (LPL) inferior parietal lobules, and corresponding correlation maps of the BOLD signal across the brain were generated (Figure 1). Finally, correlations with neuropsychological variables were probed voxelwise over the whole brain.Results
Clusters of increased functional connectivity emerged in FD patients among the main DMN hubs, as well as between the DMN and the middle temporal gyri, with a reduced anticorrelation between the midline DMN hubs and the right cerebellar Crus I and II (Figure 2). The connectivity between right inferior frontal gyrus and precuneus was significantly correlated with the results of the Corsi block tapping test (P=0.0001) (Figure 3).Discussion
The variable association of executive dysfunction and depression with the relatively widespread involvement of deep and periventricular white matter is present also in other chronic, although etiopathogenetically different, conditions, such as cerebral small vessel disease, Multiple Sclerosis (MS), late-life depression and mild cognitive impairment, and different DMN changes have been described in the above mentioned conditions. When analyzing the brain FC with reference to the four main hubs of the DMN in FD patients, we found clusters of increased connectivity in the medial frontal cortex, in the precuneus and parietal cortices bilaterally, without a significant correlation with WMH load. This result, however, is only apparently in contrast with those available in other white matter disorders, as WMH were present only in 14 out of 32 patients (43.8%), and with a low lesion load median Fazekas' score: 1). Furthermore, it should be noted that increased FC in cortical areas implicated in the DMN has been indeed previously reported in other conditions such as early stages of MS, Clinically Isolated Syndrome (CIS) and concussion, which share with FD the diffuse, although mild, involvement of WM 10-13.Conclusion
Widespread DMN changes are present in FD patients, and correlate with cognitive performance. Our results confirm the current view of a cerebral involvement in FD patients not simply associated to major cerebrovascular events, but also related to significant and diffuse functional changes.Acknowledgements
No acknowledgement found.References
1. Germain DP. Fabry disease. Orphanet J Rare Dis. 2010 Nov 22;5:30. 2. Cole AL, Lee PJ, Hughes DA, et al. Depression in adults with Fabry disease: a common and under-diagnosed problem. J Inherit Metab Dis. 2007 Nov;30(6):943-951. 3. Bolsover FE, Murphy E, Cipolotti L, et al. Cognitive dysfunction and depression in Fabry disease: a systematic review. J Inherit Metab Dis. 2014 Mar;37(2):177-187. 4. Vemuri P, Jones DT, Jack CR, Jr. Resting state functional MRI in Alzheimer's Disease. Alzheimers Res Ther. 2012 Jan 10;4(1):2. 5. Prodoehl J, Burciu RG, Vaillancourt DE. Resting state functional magnetic resonance imaging in Parkinson's disease. Curr Neurol Neurosci Rep. 2014 Jun;14(6):448. 6. Quarantelli M, Salvatore E, Giorgio SM, et al. Default-mode network changes in Huntington's disease: an integrated MRI study of functional connectivity and morphometry. PLoS One. 2013;8(8):e72159. 7. Cataldi M, Avoli M, de Villers-Sidani E. Resting state networks in temporal lobe epilepsy. Epilepsia. 2013 Dec;54(12):2048-2059. 8. Cocozza S, Pisani A, Olivo G, et al. Alterations of functional connectivity of the motor cortex in Fabry disease: An RS-fMRI study. Neurology. 2017 May 09;88(19):1822-1829. 9. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012 Jan 02;59(1):431-438. 10. Hawellek DJ, Hipp JF, Lewis CM, et al. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A. 2011 Nov 22;108(47):19066-19071. 11. Newsome MR, Li X, Lin X, et al. Functional Connectivity Is Altered in Concussed Adolescent Athletes Despite Medical Clearance to Return to Play: A Preliminary Report. Front Neurol. 2016;7:116. 12. Roosendaal SD, Schoonheim MM, Hulst HE, et al. Resting state networks change in clinically isolated syndrome. Brain. 2010 Jun;133(Pt 6):1612-1621. 13. Sharp DJ, Beckmann CF, Greenwood R, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011 Aug;134(Pt 8):2233-2247.Figures
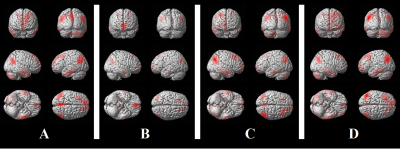
Mean
correlation maps for the four seeds chosen for the resting-state fMRI analysis,
averaged across all participants and superimposed on a 3-dimensional cortical
surface of a healthy brain in the Montreal Neurological Institute space. A: ventral portion of the precuneus; B:
medial prefrontal cortex; C: right parietal lobule; D: left parietal lobule
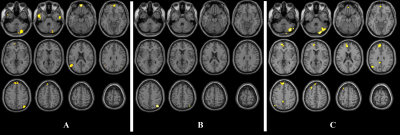
Clusters
resulting from the FD > HC between-group contrast for the seed placed at
level of the medial prefrontal cortex (A), of the ventral portion of the
precuneus (B) and of the right parietal lobule (C).
Results
are superimposed for anatomic reference to a single individual’s T1-weighted
volume in the standard Montreal Neurological Institute space, with subject’s
right at the observer’s right.
Significance
for all clusters is p < 0.05, family-wise error corrected at cluster level.
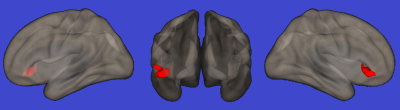
Cluster
localized at the level of right inferior frontal gyrus showing a significant
direct correlation with scores at the Corsi block-tapping test (P=0.0001).
Results is superimposed on a 3-dimensional rendering of a healthy brain in the
Montreal Neurological Institute space.